Interaction of c-Cbl with myosin IIA regulates Bleb associated macropinocytosis of Kaposi's sarcoma-associated herpesvirus
- PMID: 21203488
- PMCID: PMC3009604
- DOI: 10.1371/journal.ppat.1001238
Interaction of c-Cbl with myosin IIA regulates Bleb associated macropinocytosis of Kaposi's sarcoma-associated herpesvirus
Erratum in
- PLoS Pathog. 2011;7(2). doi:10.1371/annotation/79109603-41dd-40b8-a9ec-8df2c7fa42eb
Abstract
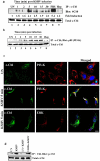
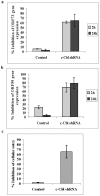
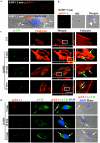
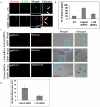
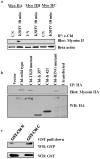
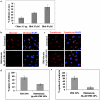
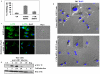
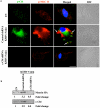
KSHV is etiologically associated with Kaposi's sarcoma (KS), an angioproliferative endothelial cell malignancy. Macropinocytosis is the predominant mode of in vitro entry of KSHV into its natural target cells, human dermal microvascular endothelial (HMVEC-d) cells. Although macropinocytosis is known to be a major route of entry for many viruses, the molecule(s) involved in the recruitment and integration of signaling early during macropinosome formation is less well studied. Here we demonstrate that tyrosine phosphorylation of the adaptor protein c-Cbl is required for KSHV induced membrane blebbing and macropinocytosis. KSHV induced the tyrosine phosphorylation of c-Cbl as early as 1 min post-infection and was recruited to the sites of bleb formation. Infection also led to an increase in the interaction of c-Cbl with PI3-K p85 in a time dependent manner. c-Cbl shRNA decreased the formation of KSHV induced membrane blebs and macropinocytosis as well as virus entry. Immunoprecipitation of c-Cbl followed by mass spectrometry identified the interaction of c-Cbl with a novel molecular partner, non-muscle myosin heavy chain IIA (myosin IIA), in bleb associated macropinocytosis. Phosphorylated c-Cbl colocalized with phospho-myosin light chain II in the interior of blebs of infected cells and this interaction was abolished by c-Cbl shRNA. Studies with the myosin II inhibitor blebbistatin demonstrated that myosin IIA is a biologically significant component of the c-Cbl signaling pathway and c-Cbl plays a new role in the recruitment of myosin IIA to the blebs during KSHV infection. Myosin II associates with actin in KSHV induced blebs and the absence of actin and myosin ubiquitination in c-Cbl ShRNA cells suggested that c-Cbl is also responsible for the ubiquitination of these proteins in the infected cells. This is the first study demonstrating the role of c-Cbl in viral entry as well as macropinocytosis, and provides the evidence that a signaling complex containing c-Cbl and myosin IIA plays a crucial role in blebbing and macropinocytosis during viral infection and suggests that targeting c-Cbl could lead to a block in KSHV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Kaposi's sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection.Proc Natl Acad Sci U S A. 2012 May 8;109(19):E1163-72. doi: 10.1073/pnas.1119592109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509030 Free PMC article.
-
CIB1 synergizes with EphrinA2 to regulate Kaposi's sarcoma-associated herpesvirus macropinocytic entry in human microvascular dermal endothelial cells.PLoS Pathog. 2014 Feb 13;10(2):e1003941. doi: 10.1371/journal.ppat.1003941. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24550731 Free PMC article.
-
Insight into the Roles of E3 Ubiquitin Ligase c-Cbl, ESCRT Machinery, and Host Cell Signaling in Kaposi's Sarcoma-Associated Herpesvirus Entry and Trafficking.J Virol. 2018 Jan 30;92(4):e01376-17. doi: 10.1128/JVI.01376-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29167336 Free PMC article. Review.
-
c-Cbl-mediated selective virus-receptor translocations into lipid rafts regulate productive Kaposi's sarcoma-associated herpesvirus infection in endothelial cells.J Virol. 2011 Dec;85(23):12410-30. doi: 10.1128/JVI.05953-11. Epub 2011 Sep 21. J Virol. 2011. PMID: 21937638 Free PMC article.
-
KSHV Entry and Trafficking in Target Cells-Hijacking of Cell Signal Pathways, Actin and Membrane Dynamics.Viruses. 2016 Nov 14;8(11):305. doi: 10.3390/v8110305. Viruses. 2016. PMID: 27854239 Free PMC article. Review.
Cited by
-
Kaposi's sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection.Proc Natl Acad Sci U S A. 2012 May 8;109(19):E1163-72. doi: 10.1073/pnas.1119592109. Epub 2012 Apr 16. Proc Natl Acad Sci U S A. 2012. PMID: 22509030 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus induces the ATM and H2AX DNA damage response early during de novo infection of primary endothelial cells, which play roles in latency establishment.J Virol. 2014 Mar;88(5):2821-34. doi: 10.1128/JVI.03126-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352470 Free PMC article.
-
The Measles Virus Receptor SLAMF1 Can Mediate Particle Endocytosis.J Virol. 2017 Mar 13;91(7):e02255-16. doi: 10.1128/JVI.02255-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28100610 Free PMC article.
-
African swine fever virus uses macropinocytosis to enter host cells.PLoS Pathog. 2012;8(6):e1002754. doi: 10.1371/journal.ppat.1002754. Epub 2012 Jun 14. PLoS Pathog. 2012. PMID: 22719252 Free PMC article.
-
Non-Muscle Myosin II A: Friend or Foe in Cancer?Int J Mol Sci. 2024 Aug 30;25(17):9435. doi: 10.3390/ijms25179435. Int J Mol Sci. 2024. PMID: 39273383 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J Virol. 2009;83:4895–4911. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

