Summary of cerebrospinal fluid routine parameters in neurodegenerative diseases
- PMID: 21188408
- PMCID: PMC3101362
- DOI: 10.1007/s00415-010-5876-x
Summary of cerebrospinal fluid routine parameters in neurodegenerative diseases
Abstract
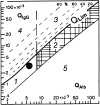
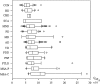
In neurodegenerative diseases, cerebrospinal fluid analysis (CSF) is predominantly performed to exclude inflammatory diseases and to perform a risk assessment in dementive disorders by measurement of tau proteins and amyloid beta peptides. However, large scale data on basic findings of CSF routine parameters are generally lacking. The objective of the study was to define a normal reference spectrum of routine CSF parameters in neurodegenerative diseases. Routine CSF parameters (white cell count, lactate and albumin concentrations, CSF/serum quotients of albumin (Q (alb)), IgG, IgA, IgM, and oligoclonal IgG bands (OCB)) were retrospectively analyzed in an academic research setting. A total of 765 patients (Alzheimer's disease (AD), Parkinson's disease (PD), Parkinson's disease dementia (PDD), vascular dementia (VD), frontotemporal lobar degeneration (FTLD), progressive supranuclear palsy (PSP), multisystem atrophy (MSA), motor neuron diseases (MND), spinocerebellar ataxia (SCA), Huntington's disease (HD)) and non-demented control groups including a group of patients with muscular disorders (MD). The main outcome measures included statistical analyses of routine CSF parameters. Mildly elevated Q (alb) were found in a small percentage of nearly all subgroups and in a higher proportion of patients with PSP, MSA, VD, PDD, and MND. With the exception of 1 MND patient, no intrathecal Ig synthesis was observed. Isolated OCBs in CSF were sometimes found in patients with neurodegenerative diseases without elevated cell counts; lactate levels were always normal. A slightly elevated Q (alb) was observed in a subgroup of patients with neurodegenerative diseases and does not exclude the diagnosis. Extensive elevation of routine parameters is not characteristic and should encourage a re-evaluation of the clinical diagnosis.
Figures

Similar articles
-
Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders.Arch Neurol. 2012 Nov;69(11):1445-52. doi: 10.1001/archneurol.2012.1654. Arch Neurol. 2012. PMID: 22925882
-
Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis.J Neurol. 2012 Apr;259(4):630-6. doi: 10.1007/s00415-011-6221-8. Epub 2011 Sep 6. J Neurol. 2012. PMID: 21898139 Free PMC article.
-
Cerebrospinal Fluid/Plasma Albumin Ratio as a Biomarker for Blood-Brain Barrier Impairment Across Neurodegenerative Dementias.J Alzheimers Dis. 2020;75(2):429-436. doi: 10.3233/JAD-200168. J Alzheimers Dis. 2020. PMID: 32280104
-
Cerebrospinal fluid proteomics and protein biomarkers in frontotemporal lobar degeneration: Current status and future perspectives.Biochim Biophys Acta. 2015 Jul;1854(7):757-68. doi: 10.1016/j.bbapap.2014.12.010. Epub 2014 Dec 17. Biochim Biophys Acta. 2015. PMID: 25526887 Review.
-
[Biomarkers for dementia and other neurodegenerative diseases : Current developments].Nervenarzt. 2016 Dec;87(12):1305-1309. doi: 10.1007/s00115-016-0238-2. Nervenarzt. 2016. PMID: 27844089 Review. German.
Cited by
-
Investigation of Inflammation in Lewy Body Dementia: A Systematic Scoping Review.Int J Mol Sci. 2023 Jul 28;24(15):12116. doi: 10.3390/ijms241512116. Int J Mol Sci. 2023. PMID: 37569491 Free PMC article.
-
Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls.PLoS One. 2018 May 10;13(5):e0197329. doi: 10.1371/journal.pone.0197329. eCollection 2018. PLoS One. 2018. PMID: 29746584 Free PMC article.
-
Inhibition of β-Glucocerebrosidase Activity Preserves Motor Unit Integrity in a Mouse Model of Amyotrophic Lateral Sclerosis.Sci Rep. 2017 Jul 12;7(1):5235. doi: 10.1038/s41598-017-05313-0. Sci Rep. 2017. PMID: 28701774 Free PMC article.
-
Oligoclonal bands in the cerebrospinal fluid of amyotrophic lateral sclerosis patients with disease-associated mutations.J Neurol. 2013 Jan;260(1):85-92. doi: 10.1007/s00415-012-6589-0. Epub 2012 Jul 1. J Neurol. 2013. PMID: 22752089 Free PMC article.
-
CSF concentrations of cAMP and cGMP are lower in patients with Creutzfeldt-Jakob disease but not Parkinson's disease and amyotrophic lateral sclerosis.PLoS One. 2012;7(3):e32664. doi: 10.1371/journal.pone.0032664. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396786 Free PMC article.
References
-
- Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. - DOI - PubMed
-
- Brettschneider J, Tumani H, Kiechle U, Muche R, Richards G, Lehmensiek V, Ludolph AC, Otto M. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS One. 2009;4:e7638. doi: 10.1371/journal.pone.0007638. - DOI - PMC - PubMed
-
- Deutsche Gesellschaft für Psychiatrie PuN, Deutsche Gesellschaft für Neurologie (2009) S3-Leitlinie Demenzen. 038/013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

