The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3
- PMID: 21167755
- PMCID: PMC3012379
- DOI: 10.1016/j.immuni.2010.11.027
The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3
Abstract
In the course of combating infectious agents, type I interferon (IFN) needs a timely downregulation mechanism to avoid detrimental overreaction. Here we showed a mechanism for restraining type I IFN responses, which relied on a HECT domain ubiquitin (Ub) E3 ligase, RAUL. RAUL limited type I IFN production by directly catalyzing lysine 48-linked polyubiquitination of both interferon regulatory factor 7 (IRF7) and IRF3 followed by proteasome-dependent degradation. Suppression of RAUL by dominant-negative RAUL or siRNA augmented both basal and virus-induced production of type I IFN, which resulted in reduced viral replication. The Kaposi's sarcoma-associated herpes virus immediate-early lytic cycle trigger protein RTA recruited this mechanism to augment its countermeasures against the host antiviral response. These results unveil a previously unrecognized "brake mechanism" for type I IFN that maintains proper low amounts of type I IFN under physiological conditions and restrains its magnitude when the antiviral response intensifies.
Figures



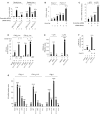
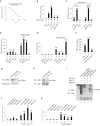

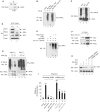
Comment in
-
CuRTAiling interferon regulatory factor signaling with the E3 ligase RAUL.Immunity. 2010 Dec 14;33(6):833-5. doi: 10.1016/j.immuni.2010.12.006. Immunity. 2010. PMID: 21168773 No abstract available.
Similar articles
-
Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF).J Biol Chem. 2011 Mar 11;286(10):7865-7872. doi: 10.1074/jbc.M110.191452. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212282 Free PMC article.
-
E3 ubiquitin ligase NEURL3 promotes innate antiviral response through catalyzing K63-linked ubiquitination of IRF7.FASEB J. 2022 Aug;36(8):e22409. doi: 10.1096/fj.202200316R. FASEB J. 2022. PMID: 35792897
-
FoxO1 negatively regulates cellular antiviral response by promoting degradation of IRF3.J Biol Chem. 2013 May 3;288(18):12596-604. doi: 10.1074/jbc.M112.444794. Epub 2013 Mar 26. J Biol Chem. 2013. PMID: 23532851 Free PMC article.
-
The Dual Role of TRIM7 in Viral Infections.Viruses. 2024 Aug 12;16(8):1285. doi: 10.3390/v16081285. Viruses. 2024. PMID: 39205259 Free PMC article. Review.
-
Rotavirus and reovirus modulation of the interferon response.J Interferon Cytokine Res. 2009 Sep;29(9):559-67. doi: 10.1089/jir.2009.0072. J Interferon Cytokine Res. 2009. PMID: 19694545 Free PMC article. Review.
Cited by
-
Regulation of cellular innate antiviral signaling by ubiquitin modification.Acta Biochim Biophys Sin (Shanghai). 2015 Mar;47(3):149-55. doi: 10.1093/abbs/gmu133. Epub 2015 Feb 3. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 25651846 Free PMC article. Review.
-
TRIM26 negatively regulates interferon-β production and antiviral response through polyubiquitination and degradation of nuclear IRF3.PLoS Pathog. 2015 Mar 12;11(3):e1004726. doi: 10.1371/journal.ppat.1004726. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25763818 Free PMC article.
-
Post-Translational Modifications of cGAS-STING: A Critical Switch for Immune Regulation.Cells. 2022 Sep 28;11(19):3043. doi: 10.3390/cells11193043. Cells. 2022. PMID: 36231006 Free PMC article. Review.
-
Zebrafish RPZ5 Degrades Phosphorylated IRF7 To Repress Interferon Production.J Virol. 2019 Oct 15;93(21):e01272-19. doi: 10.1128/JVI.01272-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413136 Free PMC article.
-
KIAA0317 regulates pulmonary inflammation through SOCS2 degradation.JCI Insight. 2019 Oct 3;4(19):e129110. doi: 10.1172/jci.insight.129110. JCI Insight. 2019. PMID: 31578312 Free PMC article.
References
-
- Barrington RE, Subler MA, Rands E, Omer CA, Miller PJ, Hundley JE, Koester SK, Troyer DA, Bearss DJ, Conner MW, et al. A farnesyltransferase inhibitor induces tumor regression in transgenic mice harboring multiple oncogenic mutations by mediating alterations in both cell cycle control and apoptosis. Mol Cell Biol. 1998;18:85–92. - PMC - PubMed
-
- Chomvarin C, Siripornmongcolchai T, Chaicumpar K, Limpaiboon T, Wongkham C, Yutanawiboonchai W. Evaluation of polymerase chain reaction, conventional and MRSA screen latex agglutination methods for detection of methicillin-resistant, - borderline and -susceptible Staphylococcus aureus. Southeast Asian J Trop Med Public Health. 2004;35:879–885. - PubMed
-
- Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature. 2004;428:1. following 486. - PubMed
-
- Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

