The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors
- PMID: 21159951
- PMCID: PMC3025500
- DOI: 10.1523/JNEUROSCI.1869-10.2010
The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors
Abstract
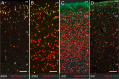
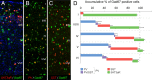
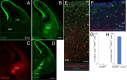
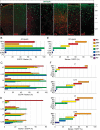
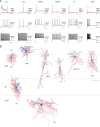
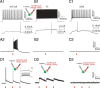
A highly diverse population of neocortical GABAergic inhibitory interneurons has been implicated in multiple functions in information processing within cortical circuits. The diversity of cortical interneurons is determined during development and primarily depends on their embryonic origins either from the medial (MGE) or the caudal (CGE) ganglionic eminences. Although MGE-derived parvalbumin (PV)- or somatostatin (SST)-expressing interneurons are well characterized, less is known about the other types of cortical GABAergic interneurons, especially those of CGE lineage, because of the lack of specific neuronal markers for these interneuron subtypes. Using a bacterial artificial chromosome transgenic mouse line, we show that, in the somatosensory cortex of the mouse, the serotonin 5-hydroxytryptamine 3A (5-HT(3A)) receptor, the only ionotropic serotonergic receptor, is expressed in most, if not all, neocortical GABAergic interneurons that do not express PV or SST. Genetic fate mapping and neurochemical profile demonstrate that 5-HT(3A)R-expressing neurons include the entire spectrum of CGE-derived interneurons. We report that, in addition to serotonergic responsiveness via 5-HT(3A)Rs, acetylcholine also depolarizes 5-HT(3A)R-expressing neurons via nicotinic receptors. 5-HT(3A)R-expressing neurons in thalamocortical (TC) recipient areas receive weak but direct monosynaptic inputs from the thalamus. TC input depolarizes a subset of TC-recipient 5-HT(3A)R neurons as strongly as fast-spiking cells, in part because of their high input resistance. Hence, fast modulation of serotonergic and cholinergic transmission may influence cortical activity through an enhancement of GABAergic synaptic transmission from 5-HT(3A)R-expressing neurons during sensory process depending on different behavioral states.
Figures

Similar articles
-
Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations.Cereb Cortex. 2010 Oct;20(10):2333-47. doi: 10.1093/cercor/bhp310. Epub 2010 Jan 18. Cereb Cortex. 2010. PMID: 20083553 Free PMC article.
-
A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala.Neuroscience. 2007 Feb 9;144(3):1015-24. doi: 10.1016/j.neuroscience.2006.10.044. Epub 2006 Dec 5. Neuroscience. 2007. PMID: 17150309 Free PMC article.
-
Interneurons Differentially Contribute to Spontaneous Network Activity in the Developing Hippocampus Dependent on Their Embryonic Lineage.J Neurosci. 2016 Mar 2;36(9):2646-62. doi: 10.1523/JNEUROSCI.4000-15.2016. J Neurosci. 2016. PMID: 26937006 Free PMC article.
-
[Cortical 5-hydroxytryptamine receptor 3A (Htr3a) positive inhibitory neurons: diversity in type and function].Sheng Li Xue Bao. 2021 Apr 25;73(2):295-305. Sheng Li Xue Bao. 2021. PMID: 33903891 Review. Chinese.
-
Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons.Dev Neurobiol. 2011 Jan 1;71(1):45-61. doi: 10.1002/dneu.20853. Dev Neurobiol. 2011. PMID: 21154909 Free PMC article. Review.
Cited by
-
Association of astrocytes with neurons and astrocytes derived from distinct progenitor domains in the subpallium.Sci Rep. 2015 Jul 20;5:12258. doi: 10.1038/srep12258. Sci Rep. 2015. PMID: 26193445 Free PMC article.
-
Ciliary neuropeptidergic signaling dynamically regulates excitatory synapses in postnatal neocortical pyramidal neurons.Elife. 2021 Mar 2;10:e65427. doi: 10.7554/eLife.65427. Elife. 2021. PMID: 33650969 Free PMC article.
-
Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits.Front Neural Circuits. 2016 Sep 29;10:76. doi: 10.3389/fncir.2016.00076. eCollection 2016. Front Neural Circuits. 2016. PMID: 27746722 Free PMC article. Review.
-
Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity.Nat Neurosci. 2013 Aug;16(8):1032-41. doi: 10.1038/nn.3459. Epub 2013 Jul 14. Nat Neurosci. 2013. PMID: 23852113 Free PMC article.
-
Brain-Wide Maps of Synaptic Input to Cortical Interneurons.J Neurosci. 2016 Apr 6;36(14):4000-9. doi: 10.1523/JNEUROSCI.3967-15.2016. J Neurosci. 2016. PMID: 27053207 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997a;19:27–37. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997b;278:474–476. - PubMed
-
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. - PMC - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
