Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production
- PMID: 21149439
- PMCID: PMC3057810
- DOI: 10.1074/jbc.M110.210047
Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production
Abstract
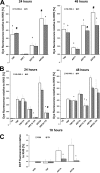
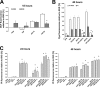
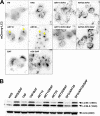
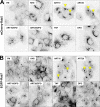
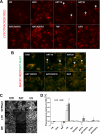
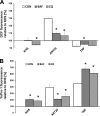
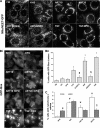
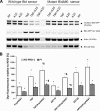
The antimalarial agent artesunate (ART) activates programmed cell death (PCD) in cancer cells in a manner dependent on the presence of iron and the generation of reactive oxygen species. In malaria parasites, ART cytotoxicity originates from interactions with heme-derived iron within the food vacuole. The analogous digestive compartment of mammalian cells, the lysosome, similarly contains high levels of redox-active iron and in response to specific stimuli can initiate mitochondrial apoptosis. We thus investigated the role of lysosomes in ART-induced PCD and determined that in MCF-7 breast cancer cells ART activates lysosome-dependent mitochondrial outer membrane permeabilization. ART impacted endolysosomal and autophagosomal compartments, inhibiting autophagosome turnover and causing perinuclear clustering of autophagosomes, early and late endosomes, and lysosomes. Lysosomal iron chelation blocked all measured parameters of ART-induced PCD, whereas lysosomal iron loading enhanced death, thus identifying lysosomal iron as the lethal source of reactive oxygen species upstream of mitochondrial outer membrane permeabilization. Moreover, lysosomal inhibitors chloroquine and bafilomycin A1 reduced ART-activated PCD, evidencing a requirement for lysosomal function during PCD signaling. ART killing did not involve activation of the BH3-only protein, Bid, yet ART enhanced TNF-mediated Bid cleavage. We additionally demonstrated the lysosomal PCD pathway in T47D and MDA-MB-231 breast cancer cells. Importantly, non-tumorigenic MCF-10A cells resisted ART-induced PCD. Together, our data suggest that ART triggers PCD via engagement of distinct, interconnected PCD pathways, with hierarchical signaling from lysosomes to mitochondria, suggesting a potential clinical use of ART for targeting lysosomes in cancer treatment.
Figures
Similar articles
-
Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin.J Biol Chem. 2014 Nov 28;289(48):33425-41. doi: 10.1074/jbc.M114.564567. Epub 2014 Oct 10. J Biol Chem. 2014. PMID: 25305013 Free PMC article.
-
The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk.Int J Cancer. 2013 Jun 1;132(11):2682-93. doi: 10.1002/ijc.27935. Epub 2012 Dec 4. Int J Cancer. 2013. PMID: 23151917
-
Lysosomal signaling enhances mitochondria-mediated photodynamic therapy in A431 cancer cells: role of iron.Photochem Photobiol. 2012 Mar-Apr;88(2):461-8. doi: 10.1111/j.1751-1097.2012.01081.x. Epub 2012 Jan 25. Photochem Photobiol. 2012. PMID: 22220628 Free PMC article.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
The lysosome as a master regulator of iron metabolism.Trends Biochem Sci. 2021 Dec;46(12):960-975. doi: 10.1016/j.tibs.2021.07.003. Epub 2021 Aug 9. Trends Biochem Sci. 2021. PMID: 34384657 Review.
Cited by
-
Current status and prospects of MOFs loaded with H2O2-related substances for ferroptosis therapy.RSC Med Chem. 2024 Jun 27;15(9):2996-3016. doi: 10.1039/d4md00261j. eCollection 2024 Sep 19. RSC Med Chem. 2024. PMID: 39309362 Review.
-
Recent advances in mitochondria-targeting theranostic agents.Exploration (Beijing). 2024 Mar 5;4(4):20230063. doi: 10.1002/EXP.20230063. eCollection 2024 Aug. Exploration (Beijing). 2024. PMID: 39175881 Free PMC article. Review.
-
Phytometabolites as modulators of breast cancer: a comprehensive review of mechanistic insights.Med Oncol. 2024 Jan 3;41(2):45. doi: 10.1007/s12032-023-02269-2. Med Oncol. 2024. PMID: 38172452 Review.
-
Mesenchymal-epithelial transition and AXL inhibitor TP-0903 sensitise triple-negative breast cancer cells to the antimalarial compound, artesunate.Sci Rep. 2024 Jan 3;14(1):425. doi: 10.1038/s41598-023-50710-3. Sci Rep. 2024. PMID: 38172210 Free PMC article.
-
The Antimalarial Drug Artesunate Mediates Selective Cytotoxicity by Upregulating HO-1 in Melanoma Cells.Biomedicines. 2023 Aug 27;11(9):2393. doi: 10.3390/biomedicines11092393. Biomedicines. 2023. PMID: 37760834 Free PMC article.
References
-
- Klayman D. L. (1985) Science 228, 1049–1055 - PubMed
-
- Efferth T., Volm M. (2005) In Vivo 19, 225–232 - PubMed
-
- Lai H., Sasaki T., Singh N. P., Messay A. (2005) Life Sci. 76, 1267–1279 - PubMed
-
- Efferth T. (2006) Curr. Drug Targets 7, 407–421 - PubMed
-
- Chen H., Sun B., Pan S., Jiang H., Sun X. (2009) Anticancer Drugs 20, 131–140 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

