Hippo signaling: growth control and beyond
- PMID: 21138973
- PMCID: PMC2998162
- DOI: 10.1242/dev.045500
Hippo signaling: growth control and beyond
Abstract
The Hippo pathway has emerged as a conserved signaling pathway that is essential for the proper regulation of organ growth in Drosophila and vertebrates. Although the mechanisms of signal transduction of the core kinases Hippo/Mst and Warts/Lats are relatively well understood, less is known about the upstream inputs of the pathway and about the downstream cellular and developmental outputs. Here, we review recently discovered mechanisms that contribute to the dynamic regulation of Hippo signaling during Drosophila and vertebrate development. We also discuss the expanding diversity of Hippo signaling functions during development, discoveries that shed light on a complex regulatory system and provide exciting new insights into the elusive mechanisms that regulate organ growth and regeneration.
Figures
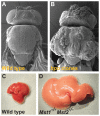
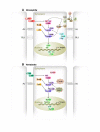
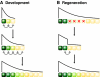
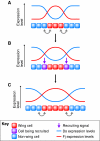
Similar articles
-
Upstream paths for Hippo signaling in Drosophila organ development.BMB Rep. 2018 Mar;51(3):134-142. doi: 10.5483/bmbrep.2018.51.3.027. BMB Rep. 2018. PMID: 29397870 Free PMC article. Review.
-
Hippo signaling: a hub of growth control, tumor suppression and pluripotency maintenance.J Genet Genomics. 2011 Oct 20;38(10):471-81. doi: 10.1016/j.jgg.2011.09.009. Epub 2011 Sep 23. J Genet Genomics. 2011. PMID: 22035868 Review.
-
Regulation of organ size: insights from the Drosophila Hippo signaling pathway.Dev Dyn. 2009 Jul;238(7):1627-37. doi: 10.1002/dvdy.21996. Dev Dyn. 2009. PMID: 19517570 Review.
-
Hippo signaling in Drosophila: recent advances and insights.Dev Dyn. 2012 Jan;241(1):3-15. doi: 10.1002/dvdy.22723. Epub 2011 Aug 25. Dev Dyn. 2012. PMID: 22174083 Free PMC article. Review.
-
The Hippo Pathway Targets Rae1 to Regulate Mitosis and Organ Size and to Feed Back to Regulate Upstream Components Merlin, Hippo, and Warts.PLoS Genet. 2016 Aug 5;12(8):e1006198. doi: 10.1371/journal.pgen.1006198. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27494403 Free PMC article.
Cited by
-
XMU-MP-1 induces growth arrest in a model human mini-organ and antagonises cell cycle-dependent paclitaxel cytotoxicity.Cell Div. 2020 Sep 17;15:11. doi: 10.1186/s13008-020-00067-0. eCollection 2020. Cell Div. 2020. PMID: 32973917 Free PMC article.
-
Signaling Pathways Governing Cardiomyocyte Differentiation.Genes (Basel). 2024 Jun 18;15(6):798. doi: 10.3390/genes15060798. Genes (Basel). 2024. PMID: 38927734 Free PMC article. Review.
-
Perfluorooctanoic acid (PFOA) promotes follicular growth and alters expression of genes that regulate the cell cycle and the Hippo pathway in cultured neonatal mouse ovaries.Toxicol Appl Pharmacol. 2022 Nov 1;454:116253. doi: 10.1016/j.taap.2022.116253. Epub 2022 Sep 21. Toxicol Appl Pharmacol. 2022. PMID: 36152675 Free PMC article.
-
siRNA Silencing of FpVtg Induces Ovarian Cell Apoptosis in Redtail Prawn, Fenneropenaeus penicillatus.Mar Biotechnol (NY). 2023 Dec;25(6):1176-1190. doi: 10.1007/s10126-023-10269-6. Epub 2023 Nov 27. Mar Biotechnol (NY). 2023. PMID: 38010485
-
TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPα function.Mol Cell. 2013 Jul 25;51(2):211-25. doi: 10.1016/j.molcel.2013.05.013. Epub 2013 Jun 13. Mol Cell. 2013. PMID: 23769673 Free PMC article.
References
-
- Adler P. N., Charlton J., Liu J. (1998). Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125, 959-968 - PubMed
-
- Affolter M., Basler K. (2007). The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat. Rev. Genet. 8, 663-674 - PubMed
-
- Ashery-Padan R., Alvarez-Bolado G., Klamt B., Gessler M., Gruss P. (1999). Fjx1, the murine homologue of the Drosophila four-jointed gene, codes for a putative secreted protein expressed in restricted domains of the developing and adult brain. Mech. Dev. 80, 213-217 - PubMed
-
- Badouel C., Gardano L., Amin N., Garg A., Rosenfeld R., Le Bihan T., McNeill H. (2009a). The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev. Cell 16, 411-420 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

