Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy
- PMID: 21084490
- PMCID: PMC3019993
- DOI: 10.1128/JVI.01701-10
Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy
Abstract
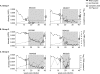
The impact of antiretroviral therapy (ART) on the genetics of simian immunodeficiency virus (SIV) or human immunodeficiency virus (HIV) populations has been incompletely characterized. We analyzed SIV genetic variation before, during, and after ART in a macaque model. Six pigtail macaques were infected with an SIV/HIV chimeric virus, RT-SHIV(mne), in which SIV reverse transcriptase (RT) was replaced by HIV-1 RT. Three animals received a short course of efavirenz (EFV) monotherapy before combination ART was started. All macaques received 20 weeks of tenofovir, emtricitabine, and EFV. Plasma virus populations were analyzed by single-genome sequencing. Population diversity was measured by average pairwise difference, and changes in viral genetics were assessed by phylogenetic and panmixia analyses. After 20 weeks of ART, viral diversity was not different from pretherapy viral diversity despite more than 10,000-fold declines in viremia, indicating that, within this range, there is no relationship between diversity and plasma viremia. In two animals with consistent SIV RNA suppression to <15 copies/ml during ART, there was no evidence of viral evolution. In contrast, in the four macaques with viremias >15 copies/ml during therapy, there was divergence between pre- and during-ART virus populations. Drug resistance mutations emerged in two of these four animals, resulting in virologic failure in the animal with the highest level of pretherapy viremia. Taken together, these findings indicate that viral diversity does not decrease with suppressive ART, that ongoing replication occurs with viremias >15 copies/ml, and that in this macaque model of ART drug resistance likely emerges as a result of incomplete suppression and preexisting drug resistance mutations.
Figures




Similar articles
-
Well-mixed plasma and tissue viral populations in RT-SHIV-infected macaques implies a lack of viral replication in the tissues during antiretroviral therapy.Retrovirology. 2015 Nov 11;12:93. doi: 10.1186/s12977-015-0212-2. Retrovirology. 2015. PMID: 26559632 Free PMC article.
-
Residual viremia in an RT-SHIV rhesus macaque HAART model marked by the presence of a predominant plasma clone and a lack of viral evolution.PLoS One. 2014 Feb 5;9(2):e88258. doi: 10.1371/journal.pone.0088258. eCollection 2014. PLoS One. 2014. PMID: 24505452 Free PMC article.
-
Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection.Retrovirology. 2007 Apr 6;4:25. doi: 10.1186/1742-4690-4-25. Retrovirology. 2007. PMID: 17417971 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
-
Considerations in the development of nonhuman primate models of combination antiretroviral therapy for studies of AIDS virus suppression, residual virus, and curative strategies.Curr Opin HIV AIDS. 2013 Jul;8(4):262-72. doi: 10.1097/COH.0b013e328361cf40. Curr Opin HIV AIDS. 2013. PMID: 23698559 Free PMC article. Review.
Cited by
-
Simian Immunodeficiency Virus SIVsab Infection of Rhesus Macaques as a Model of Complete Immunological Suppression with Persistent Reservoirs of Replication-Competent Virus: Implications for Cure Research.J Virol. 2015 Jun;89(11):6155-60. doi: 10.1128/JVI.00256-15. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833043 Free PMC article.
-
Well-mixed plasma and tissue viral populations in RT-SHIV-infected macaques implies a lack of viral replication in the tissues during antiretroviral therapy.Retrovirology. 2015 Nov 11;12:93. doi: 10.1186/s12977-015-0212-2. Retrovirology. 2015. PMID: 26559632 Free PMC article.
-
Animal Models for HIV Cure Research.Front Immunol. 2016 Jan 28;7:12. doi: 10.3389/fimmu.2016.00012. eCollection 2016. Front Immunol. 2016. PMID: 26858716 Free PMC article. Review.
-
A spatio-temporal assessment of simian/human immunodeficiency virus (SHIV) evolution reveals a highly dynamic process within the host.PLoS Pathog. 2017 May 25;13(5):e1006358. doi: 10.1371/journal.ppat.1006358. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542550 Free PMC article.
-
HIV reservoirs and strategies for eradication.Curr HIV/AIDS Rep. 2012 Mar;9(1):5-15. doi: 10.1007/s11904-011-0108-2. Curr HIV/AIDS Rep. 2012. PMID: 22249405 Review.
References
-
- Achaz, G., S. Palmer, M. Kearney, F. Maldarelli, J. W. Mellors, J. M. Coffin, and J. Wakeley. 2004. A robust measure of HIV-1 population turnover within chronically infected individuals. Mol. Biol. Evol. 21:1902-1912. - PubMed
-
- Ambrose, Z., V. Boltz, S. Palmer, J. M. Coffin, S. H. Hughes, and V. N. Kewalramani. 2004. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J. Virol. 78:13553-13561. - PMC - PubMed
-
- Ambrose, Z., S. Palmer, V. F. Boltz, M. Kearney, K. Larsen, P. Polacino, L. Flanary, K. Oswald, M. Piatak, Jr., J. Smedley, W. Shao, N. Bischofberger, F. Maldarelli, J. T. Kimata, J. W. Mellors, S. L. Hu, J. M. Coffin, J. D. Lifson, and V. N. KewalRamani. 2007. Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy. J. Virol. 81:12145-12155. - PMC - PubMed
-
- Bailey, J. R., A. R. Sedaghat, T. Kieffer, T. Brennan, P. K. Lee, M. Wind-Rotolo, C. M. Haggerty, A. R. Kamireddi, Y. Liu, J. Lee, D. Persaud, J. E. Gallant, J. Cofrancesco, Jr., T. C. Quinn, C. O. Wilke, S. C. Ray, J. D. Siliciano, R. E. Nettles, and R. F. Siliciano. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80:6441-6457. - PMC - PubMed
-
- Benito, J. M., M. Lopez, S. Lozano, P. Martinez, J. Gonzalez-Lahoz, and V. Soriano. 2004. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res. Hum. Retroviruses 20:227-233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

