Activation of plasmacytoid dendritic cells by Kaposi's sarcoma-associated herpesvirus
- PMID: 20980519
- PMCID: PMC3020034
- DOI: 10.1128/JVI.01007-10
Activation of plasmacytoid dendritic cells by Kaposi's sarcoma-associated herpesvirus
Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is associated with multiple human malignancies, including Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Following primary infection, KSHV typically goes through a brief period of lytic replication prior to the establishment of latency. Plasmacytoid dendritic cells (pDCs) are the major producers of type 1 interferon (IFN), primarily in response to virus infection. Toll-like receptors (TLRs) are key components of the innate immune system, and they serve as pathogen recognition receptors that stimulate the host antiviral response. pDCs express exclusively TLR7 and TLR9, and it is through these TLRs that the type 1 interferon response is activated in pDCs. Currently, it is not known whether KSHV is recognized by pDCs and whether activation of pDCs occurs in response to KSHV infection. We now report evidence that KSHV can infect human pDCs and that pDCs are activated upon KSHV infection, as measured by upregulation of CD83 and CD86 and by IFN-α secretion. We further show that induction of IFN-α occurs through activation of TLR9 signaling and that a TLR9 inhibitor diminishes the production and secretion of IFN-α by KSHV-infected pDCs.
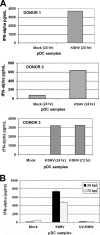
Figures
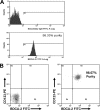
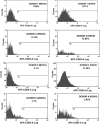
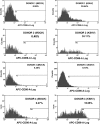

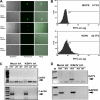

Similar articles
-
Endosomal Toll-Like Receptors 7 and 9 Cooperate in Detection of Murine Gammaherpesvirus 68 Infection.J Virol. 2019 Jan 17;93(3):e01173-18. doi: 10.1128/JVI.01173-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429335 Free PMC article.
-
Plasmacytoid dendritic cells in skin lesions of classic Kaposi's sarcoma.Arch Dermatol Res. 2016 Sep;308(7):487-92. doi: 10.1007/s00403-016-1671-6. Epub 2016 Jul 2. Arch Dermatol Res. 2016. PMID: 27372661
-
RIG-I Detects Kaposi's Sarcoma-Associated Herpesvirus Transcripts in a RNA Polymerase III-Independent Manner.mBio. 2018 Jul 3;9(4):e00823-18. doi: 10.1128/mBio.00823-18. mBio. 2018. PMID: 29970461 Free PMC article.
-
Interplay between Kaposi's sarcoma-associated herpesvirus and the innate immune system.Cytokine Growth Factor Rev. 2014 Oct;25(5):597-609. doi: 10.1016/j.cytogfr.2014.06.001. Epub 2014 Jun 21. Cytokine Growth Factor Rev. 2014. PMID: 25037686 Free PMC article. Review.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
Cited by
-
Learning from the messengers: innate sensing of viruses and cytokine regulation of immunity - clues for treatments and vaccines.Viruses. 2013 Jan 31;5(2):470-527. doi: 10.3390/v5020470. Viruses. 2013. PMID: 23435233 Free PMC article. Review.
-
Viral interferon regulatory factors decrease the induction of type I and type II interferon during rhesus macaque rhadinovirus infection.J Virol. 2012 Feb;86(4):2197-211. doi: 10.1128/JVI.05047-11. Epub 2011 Dec 7. J Virol. 2012. PMID: 22156526 Free PMC article.
-
Mesenchymal stem cells detect and defend against gammaherpesvirus infection via the cGAS-STING pathway.Sci Rep. 2015 Jan 16;5:7820. doi: 10.1038/srep07820. Sci Rep. 2015. PMID: 25592282 Free PMC article.
-
KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression.Autophagy. 2016 Dec;12(12):2311-2325. doi: 10.1080/15548627.2016.1235122. Epub 2016 Oct 7. Autophagy. 2016. PMID: 27715410 Free PMC article.
-
AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV.Front Immunol. 2013 Jan 7;3:401. doi: 10.3389/fimmu.2012.00401. eCollection 2012. Front Immunol. 2013. PMID: 23316192 Free PMC article.
References
-
- Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. - PubMed
-
- Akira, S., and S. Sato. 2003. Toll-like receptors and their signaling mechanisms. Scand. J. Infect. Dis. 35:555-562. - PubMed
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI077454/AI/NIAID NIH HHS/United States
- CA096500/CA/NCI NIH HHS/United States
- P20 RR015577/RR/NCRR NIH HHS/United States
- T32-AI007419/AI/NIAID NIH HHS/United States
- R01 AI080432/AI/NIAID NIH HHS/United States
- R01 AI077454/AI/NIAID NIH HHS/United States
- DE018304/DE/NIDCR NIH HHS/United States
- T32 AI007419/AI/NIAID NIH HHS/United States
- R01 DE018304/DE/NIDCR NIH HHS/United States
- F32-AI078735/AI/NIAID NIH HHS/United States
- AI080432/AI/NIAID NIH HHS/United States
- DE018281/DE/NIDCR NIH HHS/United States
- R01 DE018281/DE/NIDCR NIH HHS/United States
- F32 AI078735/AI/NIAID NIH HHS/United States
- R01 CA096500/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources

