An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury
- PMID: 20962258
- PMCID: PMC3020135
- DOI: 10.4049/jimmunol.1002330
An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury
Erratum in
- J Immunol. 2011 Feb 1;186(3):1880
Abstract
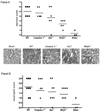
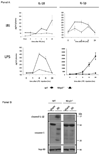
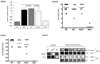
Cytoplasmic innate immune receptors are important therapeutic targets for diseases associated with overproduction of proinflammatory cytokines. One cytoplasmic receptor complex, the Nlrp3 inflammasome, responds to an extensive array of molecules associated with cellular stress. Under normal conditions, Nlrp3 is autorepressed, but in the presence of its ligands, it oligomerizes, recruits apoptosis-associated speck-like protein containing a caspase recruitment domain (Asc), and triggers caspase 1 activation and the maturation of proinflammatory cytokines such as IL-1β and IL-18. Because ischemic tissue injury provides a potential source for Nlrp3 ligands, our study compared and contrasted the effects of renal ischemia in wild-type mice and mice deficient in components of the Nlrp3 inflammasome (Nlrp3(-/-) and Asc(-/-) mice). To examine the role of the inflammasome in renal ischemia-reperfusion injury (IRI) we also tested its downstream targets caspase 1, IL-1β, and IL-18. Both Nlrp3 and Asc were highly expressed in renal tubular epithelium of humans and mice, and the absence of Nlrp3, but not Asc or the downstream inflammasome targets, dramatically protected from kidney IRI. We conclude that Nlrp3 contributes to renal IRI by a direct effect on renal tubular epithelium and that this effect is independent of inflammasome-induced proinflammatory cytokine production.
Figures




Similar articles
-
NLRP3 inflammasome-mitochondrion loop in autism spectrum disorder.Free Radic Biol Med. 2024 Nov 20;225:581-594. doi: 10.1016/j.freeradbiomed.2024.10.297. Epub 2024 Oct 19. Free Radic Biol Med. 2024. PMID: 39433111
-
Interleukin-38 ameliorates myocardial Ischemia-Reperfusion injury via inhibition of NLRP3 inflammasome activation in fibroblasts through the IL-1R8/SYK axis.Int Immunopharmacol. 2024 Dec 25;143(Pt 2):113428. doi: 10.1016/j.intimp.2024.113428. Epub 2024 Oct 23. Int Immunopharmacol. 2024. PMID: 39447412
-
BAK ameliorated cerebral infarction/ischemia-reperfusion injury by activating AMPK/Nrf2 to inhibit TXNIP/NLRP3/caspase-1 axis.Neurosci Lett. 2025 Jan 1;844:138037. doi: 10.1016/j.neulet.2024.138037. Epub 2024 Nov 6. Neurosci Lett. 2025. PMID: 39515657
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Recent advances in NLRP3 inflammasome in corneal diseases: Preclinical insights and therapeutic implications.Ocul Surf. 2024 Oct;34:392-405. doi: 10.1016/j.jtos.2024.09.007. Epub 2024 Sep 30. Ocul Surf. 2024. PMID: 39357820 Review.
Cited by
-
The NLRP3 Inflammasome as a novel player of the intercellular crosstalk in metabolic disorders.Mediators Inflamm. 2013;2013:678627. doi: 10.1155/2013/678627. Epub 2013 Jun 13. Mediators Inflamm. 2013. PMID: 23843683 Free PMC article. Review.
-
Canonical and non-canonical functions of NLRP3.J Adv Res. 2023 Nov;53:137-151. doi: 10.1016/j.jare.2023.01.001. Epub 2023 Jan 4. J Adv Res. 2023. PMID: 36610670 Free PMC article. Review.
-
The immune system and kidney disease: basic concepts and clinical implications.Nat Rev Immunol. 2013 Oct;13(10):738-53. doi: 10.1038/nri3523. Epub 2013 Sep 16. Nat Rev Immunol. 2013. PMID: 24037418 Review.
-
Mitochondrial antiviral signaling protein: a potential therapeutic target in renal disease.Front Immunol. 2023 Oct 12;14:1266461. doi: 10.3389/fimmu.2023.1266461. eCollection 2023. Front Immunol. 2023. PMID: 37901251 Free PMC article. Review.
-
Psoralen Alleviates Renal Fibrosis by Attenuating Inflammasome-Dependent NLRP3 Activation and Epithelial-Mesenchymal Transition in a Mouse Unilateral Ureteral Obstruction Model.Int J Mol Sci. 2023 Aug 24;24(17):13171. doi: 10.3390/ijms241713171. Int J Mol Sci. 2023. PMID: 37685978 Free PMC article.
References
-
- Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8:465–479. - PubMed
-
- Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. - PubMed
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
-
- Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003;171:6154–6163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

