Eaten alive: a history of macroautophagy
- PMID: 20811353
- PMCID: PMC3616322
- DOI: 10.1038/ncb0910-814
Eaten alive: a history of macroautophagy
Abstract
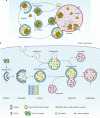
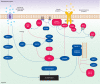
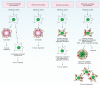
Macroautophagy (hereafter autophagy), or 'self-eating', is a conserved cellular pathway that controls protein and organelle degradation, and has essential roles in survival, development and homeostasis. Autophagy is also integral to human health and is involved in physiology, development, lifespan and a wide range of diseases, including cancer, neurodegeneration and microbial infection. Although research on this topic began in the late 1950s, substantial progress in the molecular study of autophagy has taken place during only the past 15 years. This review traces the key findings that led to our current molecular understanding of this complex process.
Figures
Similar articles
-
Chaperone-mediated autophagy: the heretofore untold story of J. Fred "Paulo" Dice. Interview by Daniel J. Klionsky.Autophagy. 2009 Nov;5(8):1079-84. doi: 10.4161/auto.5.8.9476. Epub 2009 Nov 21. Autophagy. 2009. PMID: 19617700
-
A brief history of autophagy from cell biology to physiology and disease.Nat Cell Biol. 2018 May;20(5):521-527. doi: 10.1038/s41556-018-0092-5. Epub 2018 Apr 23. Nat Cell Biol. 2018. PMID: 29686264 Review.
-
Autophagy researchers.Autophagy. 2014 Mar;10(3):393-6. doi: 10.4161/auto.27581. Autophagy. 2014. PMID: 24721973 Free PMC article. No abstract available.
-
From the urea cycle to autophagy: Alfred J. Meijer.Autophagy. 2011 Aug;7(8):805-13. doi: 10.4161/auto.7.8.15192. Epub 2011 Aug 1. Autophagy. 2011. PMID: 21389787
-
The significance of macroautophagy in health and disease.Folia Morphol (Warsz). 2013 May;72(2):87-93. doi: 10.5603/fm.2013.0015. Folia Morphol (Warsz). 2013. PMID: 23740493 Review.
Cited by
-
Targeting programmed cell death in metabolic dysfunction-associated fatty liver disease (MAFLD): a promising new therapy.Cell Mol Biol Lett. 2021 May 7;26(1):17. doi: 10.1186/s11658-021-00254-z. Cell Mol Biol Lett. 2021. PMID: 33962586 Free PMC article. Review.
-
LC3B Binds to the Autophagy Protease ATG4b with High Affinity Using a Bipartite Interface.Biochemistry. 2022 Nov 1;61(21):2295-2302. doi: 10.1021/acs.biochem.2c00482. Epub 2022 Oct 20. Biochemistry. 2022. PMID: 36264309 Free PMC article.
-
Lipopolysaccharide induction of autophagy is associated with enhanced bactericidal activity in Dictyostelium discoideum.Biochem Biophys Res Commun. 2012 Jun 8;422(3):417-22. doi: 10.1016/j.bbrc.2012.05.006. Epub 2012 May 7. Biochem Biophys Res Commun. 2012. PMID: 22575510 Free PMC article.
-
Autophagosome dynamics in neurodegeneration at a glance.J Cell Sci. 2015 Apr 1;128(7):1259-67. doi: 10.1242/jcs.161216. J Cell Sci. 2015. PMID: 25829512 Free PMC article. Review.
-
Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress.Autophagy. 2012 Feb 1;8(2):236-51. doi: 10.4161/auto.8.2.18600. Epub 2012 Feb 1. Autophagy. 2012. PMID: 22240589 Free PMC article.
References
-
- de Duve C, Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

