Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells
- PMID: 20805998
- PMCID: PMC2929211
- DOI: 10.1371/journal.pone.0012445
Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells
Abstract
Background: Current management of patients diagnosed with prostate cancer (PCa) is very effective; however, tumor recurrence with Castrate Resistant Prostate Cancer (CRPC) and subsequent metastasis lead to poor survival outcome, suggesting that there is a dire need for novel mechanistic understanding of tumor recurrence, which would be critical for designing novel therapies. The recurrence and the metastasis of PCa are tightly linked with the biology of prostate cancer stem cells or cancer-initiating cells that is reminiscent of the acquisition of Epithelial to Mesenchymal Transition (EMT) phenotype. Increasing evidence suggests that EMT-type cells share many biological characteristics with cancer stem-like cells.
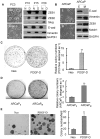
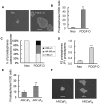
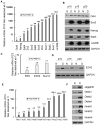
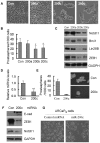
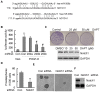
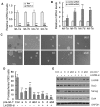
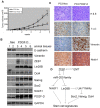
Methodology/principal findings: In this study, we found that PCa cells with EMT phenotype displayed stem-like cell features characterized by increased expression of Sox2, Nanog, Oct4, Lin28B and/or Notch1, consistent with enhanced clonogenic and sphere (prostasphere)-forming ability and tumorigenecity in mice, which was associated with decreased expression of miR-200 and/or let-7 family. Reversal of EMT by re-expression of miR-200 inhibited prostasphere-forming ability of EMT-type cells and reduced the expression of Notch1 and Lin28B. Down-regulation of Lin28B increased let-7 expression, which was consistent with repressed self-renewal capability.
Conclusions/significance: These results suggest that miR-200 played a pivotal role in linking the characteristics of cancer stem-like cells with EMT-like cell signatures in PCa. Selective elimination of cancer stem-like cells by reversing the EMT phenotype to Mesenchymal-Epithelial Transition (MET) phenotype using novel agents would be useful for the prevention of tumor recurrence especially by eliminating those cells that are the "Root Cause" of tumor development and recurrence.
Conflict of interest statement
Figures

Similar articles
-
miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells.Stem Cells. 2009 Aug;27(8):1712-21. doi: 10.1002/stem.101. Stem Cells. 2009. PMID: 19544444 Free PMC article.
-
Different Effects of BORIS/CTCFL on Stemness Gene Expression, Sphere Formation and Cell Survival in Epithelial Cancer Stem Cells.PLoS One. 2015 Jul 17;10(7):e0132977. doi: 10.1371/journal.pone.0132977. eCollection 2015. PLoS One. 2015. PMID: 26185996 Free PMC article.
-
Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells.Cell Tissue Res. 2014 Dec;358(3):763-78. doi: 10.1007/s00441-014-2001-y. Epub 2014 Oct 9. Cell Tissue Res. 2014. PMID: 25296715
-
MicroRNAs and epithelial-mesenchymal transition in prostate cancer.Oncotarget. 2016 Oct 11;7(41):67597-67611. doi: 10.18632/oncotarget.11708. Oncotarget. 2016. PMID: 27588490 Free PMC article. Review.
-
The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies.J Mammary Gland Biol Neoplasia. 2009 Mar;14(1):29-43. doi: 10.1007/s10911-009-9110-3. Epub 2009 Feb 26. J Mammary Gland Biol Neoplasia. 2009. PMID: 19242781 Review.
Cited by
-
Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice.Sci Rep. 2016 Aug 31;6:32062. doi: 10.1038/srep32062. Sci Rep. 2016. PMID: 27576789 Free PMC article.
-
Reversal of transforming growth factor-β induced epithelial-to-mesenchymal transition and the ZEB proteins.Fibrogenesis Tissue Repair. 2012 Jun 6;5(Suppl 1):S28. doi: 10.1186/1755-1536-5-S1-S28. eCollection 2012. Fibrogenesis Tissue Repair. 2012. PMID: 23259633 Free PMC article.
-
Histone deacetylase inhibitors induce epithelial-to-mesenchymal transition in prostate cancer cells.PLoS One. 2012;7(9):e45045. doi: 10.1371/journal.pone.0045045. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024790 Free PMC article.
-
Epithelial-to-mesenchymal transition in cancer: complexity and opportunities.Front Med. 2018 Aug;12(4):361-373. doi: 10.1007/s11684-018-0656-6. Epub 2018 Jul 24. Front Med. 2018. PMID: 30043221 Free PMC article. Review.
-
Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins?Cancers (Basel). 2011 Feb 21;3(1):716-29. doi: 10.3390/cancers30100716. Cancers (Basel). 2011. PMID: 21643534 Free PMC article.
References
-
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. - PubMed
-
- Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. - PubMed
-
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, ntosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

