Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling
- PMID: 20716764
- PMCID: PMC2928148
- DOI: 10.1126/scisignal.2000955
Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling
Abstract
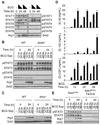
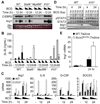
Nitric oxide (NO) produced by macrophages is toxic to host tissues and invading pathogens, and its regulation is essential to suppress host cytotoxicity. Macrophage arginase 1 (Arg1) competes with NO synthases for arginine, a substrate common to both types of enzymes, to inhibit NO production. Two signal transduction pathways control the production of Arg1 in macrophages: One pathway dependent on the Toll-like receptor adaptor protein myeloid differentiation marker 88 (MyD88) induces the expression of Arg1 during intracellular infections, whereas another pathway, which depends on signal transducer and activator of transcription 6 (STAT6), is required for Arg1 expression in alternatively activated macrophages. We found that mycobacteria-infected macrophages produced soluble factors, including interleukin-6 (IL-6), IL-10, and granulocyte colony-stimulating factor (G-CSF), that induced expression of Arg1 in an autocrine-paracrine manner. Arg1 expression was controlled by the MyD88-dependent production of these cytokines rather than by cell-intrinsic MyD88 signaling to Arg1. Our study revealed that the MyD88-dependent pathway that induced the expression of Arg1 after infection by mycobacteria required STAT3 activation and that this pathway may cause the development of an immunosuppressive niche in granulomas because of the induced production of Arg1 in surrounding uninfected macrophages.
Figures


Similar articles
-
Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control.PLoS Pathog. 2011 Sep;7(9):e1002236. doi: 10.1371/journal.ppat.1002236. Epub 2011 Sep 8. PLoS Pathog. 2011. PMID: 21931552 Free PMC article.
-
Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens.Nat Immunol. 2008 Dec;9(12):1399-406. doi: 10.1038/ni.1671. Epub 2008 Nov 2. Nat Immunol. 2008. PMID: 18978793 Free PMC article.
-
Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression.PLoS Pathog. 2012 Jan;8(1):e1002417. doi: 10.1371/journal.ppat.1002417. Epub 2012 Jan 19. PLoS Pathog. 2012. PMID: 22275864 Free PMC article.
-
Growth factor and Th2 cytokine signaling pathways converge at STAT6 to promote arginase expression in progressive experimental visceral leishmaniasis.PLoS Pathog. 2014 Jun 26;10(6):e1004165. doi: 10.1371/journal.ppat.1004165. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24967908 Free PMC article.
-
The role of myeloid cell activation and arginine metabolism in the pathogenesis of virus-induced diseases.Front Immunol. 2014 Sep 8;5:428. doi: 10.3389/fimmu.2014.00428. eCollection 2014. Front Immunol. 2014. PMID: 25250029 Free PMC article. Review.
Cited by
-
Quantifying crosstalk among interferon-γ, interleukin-12, and tumor necrosis factor signaling pathways within a TH1 cell model.Sci Signal. 2012 Apr 17;5(220):ra32. doi: 10.1126/scisignal.2002657. Sci Signal. 2012. PMID: 22510470 Free PMC article.
-
Human arginase 1, a Jack of all trades?3 Biotech. 2022 Oct;12(10):264. doi: 10.1007/s13205-022-03326-9. Epub 2022 Sep 7. 3 Biotech. 2022. PMID: 36082360 Free PMC article. Review.
-
A Trypanosoma brucei kinesin heavy chain promotes parasite growth by triggering host arginase activity.PLoS Pathog. 2013 Oct;9(10):e1003731. doi: 10.1371/journal.ppat.1003731. Epub 2013 Oct 31. PLoS Pathog. 2013. PMID: 24204274 Free PMC article.
-
Advanced age impairs macrophage polarization.J Interferon Cytokine Res. 2012 Jan;32(1):18-26. doi: 10.1089/jir.2011.0058. Epub 2011 Dec 16. J Interferon Cytokine Res. 2012. PMID: 22175541 Free PMC article.
-
The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria?Clin Dev Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. Epub 2012 Jul 3. Clin Dev Immunol. 2012. PMID: 22811737 Free PMC article. Review.
References
-
- Skold M, Behar SM. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J. Immunol. 2008;181:6349–6360. - PubMed
-
- Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399–407. - PubMed
-
- Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

