Bub1 and CENP-F can contribute to Kaposi's sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores
- PMID: 20660191
- PMCID: PMC2937805
- DOI: 10.1128/JVI.00713-10
Bub1 and CENP-F can contribute to Kaposi's sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores
Abstract
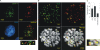
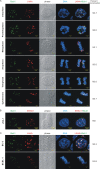
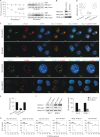
The latency-associated nuclear antigen (LANA) encoded by Kaposi's sarcoma-associated herpesvirus (KSHV) is critical for segregation of viral episomes to progeny nuclei and allows for maintenance of the viral genome in newly divided daughter cells. LANA binds to KSHV terminal repeat (TR) DNA and simultaneously associates with chromatin-bound cellular proteins. This process tethers the viral episomes to host chromosomes. However, the mechanism of tethering is complex and involves multiple protein-protein interactions. Our previous proteomics studies which showed the association of LANA with centromeric protein F (CENP-F) prompted us to further study whether LANA targets centromeric proteins for persistence of KSHV episomes during cell division. Here we show that LANA colocalized with CENP-F as speckles, some of which are paired at centromeric regions of a subset of chromosomes in KSHV-infected JSC-1 cells. We also confirm that both the amino and carboxy termini of LANA can bind to CENP-F. Moreover, LANA associated with another kinetochore protein, Bub1 (budding uninhibited by benzimidazole 1), which is known to form a complex with CENP-F. Importantly, we demonstrated the dynamic association of LANA and Bub1/CENP-F and the colocalization between Bub1, LANA, and the KSHV episome tethered to the host chromosome using fluorescence in situ hybridization (FISH). Knockdown of Bub1 expression by lentivirus-delivered short hairpin RNA (shRNA) dramatically reduced the number of KSHV genome copies, whereas no dramatic effect was seen with CENP-F knockdown. Therefore, the interaction between LANA and the kinetochore proteins CENP-F and Bub1 is important for KSHV genome tethering and its segregation to new daughter cells, with Bub1 potentially playing a more critical role in the long-term persistence of the viral genome in the infected cell.
Figures


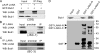



Similar articles
-
Bub1 in Complex with LANA Recruits PCNA To Regulate Kaposi's Sarcoma-Associated Herpesvirus Latent Replication and DNA Translesion Synthesis.J Virol. 2015 Oct;89(20):10206-18. doi: 10.1128/JVI.01524-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223641 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus LANA-Adjacent Regions with Distinct Functions in Episome Segregation or Maintenance.J Virol. 2019 Mar 5;93(6):e02158-18. doi: 10.1128/JVI.02158-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626680 Free PMC article.
-
Identification of Kaposi's sarcoma-associated herpesvirus LANA regions important for episome segregation, replication, and persistence.J Virol. 2013 Nov;87(22):12270-83. doi: 10.1128/JVI.01243-13. Epub 2013 Sep 4. J Virol. 2013. PMID: 24006437 Free PMC article.
-
The latency-associated nuclear antigen, a multifunctional protein central to Kaposi's sarcoma-associated herpesvirus latency.Future Microbiol. 2011 Dec;6(12):1399-413. doi: 10.2217/fmb.11.137. Future Microbiol. 2011. PMID: 22122438 Free PMC article. Review.
-
KSHV LANA--the master regulator of KSHV latency.Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
Cited by
-
The chromatin landscape of Kaposi's sarcoma-associated herpesvirus.Viruses. 2013 May 23;5(5):1346-73. doi: 10.3390/v5051346. Viruses. 2013. PMID: 23698402 Free PMC article. Review.
-
Epstein-Barr virus enhances genome maintenance of Kaposi sarcoma-associated herpesvirus.Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):E11379-E11387. doi: 10.1073/pnas.1810128115. Epub 2018 Nov 14. Proc Natl Acad Sci U S A. 2018. PMID: 30429324 Free PMC article.
-
Molecular Biology of KSHV in Relation to HIV/AIDS-Associated Oncogenesis.Cancer Treat Res. 2019;177:23-62. doi: 10.1007/978-3-030-03502-0_2. Cancer Treat Res. 2019. PMID: 30523620 Free PMC article.
-
KSHV episome tethering sites on host chromosomes and regulation of latency-lytic switch by CHD4.Cell Rep. 2022 May 10;39(6):110788. doi: 10.1016/j.celrep.2022.110788. Cell Rep. 2022. PMID: 35545047 Free PMC article.
-
Hitchhiking of Viral Genomes on Cellular Chromosomes.Annu Rev Virol. 2019 Sep 29;6(1):275-296. doi: 10.1146/annurev-virology-092818-015716. Epub 2019 Jul 5. Annu Rev Virol. 2019. PMID: 31283444 Free PMC article. Review.
References
-
- Ambroziak, J. A., D. J. Blackbourn, B. G. Herndier, R. G. Glogau, J. H. Gullett, A. R. McDonald, E. T. Lennette, and J. A. Levy. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268:582-583. - PubMed
-
- An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862-3874. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
-
- Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, V. Joukov, J. C. Walter, K. Luger, and K. M. Kaye. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856-861. - PubMed
-
- Boshoff, C. 2003. Kaposi virus scores cancer coup. Nat. Med. 9:261-262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 CA126182-03/CA/NCI NIH HHS/United States
- R01 CA091792/CA/NCI NIH HHS/United States
- CA137894/CA/NCI NIH HHS/United States
- CA108461/CA/NCI NIH HHS/United States
- CA138434/CA/NCI NIH HHS/United States
- CA091792/CA/NCI NIH HHS/United States
- K99 CA126182/CA/NCI NIH HHS/United States
- AI067037/AI/NIAID NIH HHS/United States
- CA115299/CA/NCI NIH HHS/United States
- CA126182/CA/NCI NIH HHS/United States
- R01 CA138434/CA/NCI NIH HHS/United States
- R00 CA126182/CA/NCI NIH HHS/United States
- R01 AI067037/AI/NIAID NIH HHS/United States
- T32 CA115299/CA/NCI NIH HHS/United States
- R00 CA126182-04/CA/NCI NIH HHS/United States
- R01 CA108461/CA/NCI NIH HHS/United States
- R01 CA137894/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

