Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties
- PMID: 20629994
- PMCID: PMC3822625
- DOI: 10.1111/j.1582-4934.2010.01120.x
Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties
Abstract
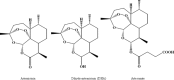
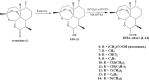
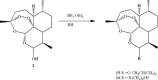
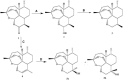
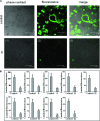
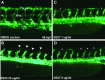
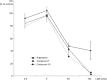
Artemisinins are plant products with a wide range of medicinal applications. Most prominently, artesunate is a well tolerated and effective drug for treating malaria, but is also active against several protozoal and schistosomal infections, and additionally exhibits anti-angiogenic, anti-tumorigenic and anti-viral properties. The array of activities of the artemisinins, and the recent emergence of malaria resistance to artesunate, prompted us to synthesize and evaluate several novel artemisinin-like derivatives. Sixteen distinct derivatives were therefore synthesized and the in vitro cytotoxic effects of each were tested with different cell lines. The in vivo anti-angiogenic properties were evaluated using a zebrafish embryo model. We herein report the identification of several novel artemisinin-like compounds that are easily synthesized, stable at room temperature, may overcome drug-resistance pathways and are more active in vitro and in vivo than the commonly used artesunate. These promising findings raise the hopes of identifying safer and more effective strategies to treat a range of infections and cancer.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures

Similar articles
-
Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin.Phytomedicine. 2011 Aug 15;18(11):959-69. doi: 10.1016/j.phymed.2011.06.008. Epub 2011 Aug 9. Phytomedicine. 2011. PMID: 21831619
-
Is artemisinin the only antiplasmodial compound in the Artemisia annua tea infusion? An in vitro study.Planta Med. 2013 Apr;79(6):468-70. doi: 10.1055/s-0032-1328324. Epub 2013 Mar 19. Planta Med. 2013. PMID: 23512495
-
Artemisia annua extracts, artemisinin and 1,8-cineole, prevent fruit infestation by a major, cosmopolitan pest of apples.Pharm Biol. 2011 Jun;49(6):563-8. doi: 10.3109/13880209.2010.528433. Epub 2011 Mar 9. Pharm Biol. 2011. PMID: 21385092
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer.Molecules. 2010 Apr 29;15(5):3135-70. doi: 10.3390/molecules15053135. Molecules. 2010. PMID: 20657468 Free PMC article. Review.
Cited by
-
Synthesis and Evaluation of Artemisinin-Based Hybrid and Dimer Derivatives as Antimelanoma Agents.ACS Omega. 2019 Dec 27;5(1):243-251. doi: 10.1021/acsomega.9b02600. eCollection 2020 Jan 14. ACS Omega. 2019. PMID: 31956771 Free PMC article.
-
Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics.Acta Pharm Sin B. 2021 Feb;11(2):322-339. doi: 10.1016/j.apsb.2020.09.001. Epub 2020 Sep 8. Acta Pharm Sin B. 2021. PMID: 33643815 Free PMC article. Review.
-
Development of Anticancer Agents from Plant-Derived Sesquiterpene Lactones.Curr Med Chem. 2016;23(23):2397-420. doi: 10.2174/0929867323666160510123255. Curr Med Chem. 2016. PMID: 27160533 Free PMC article. Review.
-
Development of artemisinin compounds for cancer treatment.Invest New Drugs. 2013 Feb;31(1):230-46. doi: 10.1007/s10637-012-9873-z. Epub 2012 Aug 31. Invest New Drugs. 2013. PMID: 22935909 Review.
-
Treatment of Iron-Loaded Veterinary Sarcoma by Artemisia annua.Nat Prod Bioprospect. 2014 Apr;4(2):113-8. doi: 10.1007/s13659-014-0013-7. Epub 2014 Apr 12. Nat Prod Bioprospect. 2014. PMID: 24859473 Free PMC article.
References
-
- Haynes RK, Chan HW, Lung CM, et al. Artesunate and dihydroartemisinin (DHA): unusual decomposition products formed under mild conditions and comments on the fitness of DHA as an Antimalarial drug. Chem Med Chem. 2007;2:1448–63. - PubMed
-
- Jansen FH, Soomro SA. Chemical instability determines the biological action of the artemisinins. Curr Med Chem. 2007;14:3243–59. - PubMed
-
- Utzinger J, Xiao SH, Tanner M, et al. Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs. 2007;8:105–16. - PubMed
-
- Shu-Hua X, Utzinger J, Chollet J, et al. Effect of artemether administered alone or in combination with praziquantel to mice infected with Plasmodium berghei or Schistosoma mansoni or both. Int J Pararasitol. 2006;36:957–64. - PubMed
-
- Efferth T. Mechanistic perspectives for 1, 2, 4-trioxanes in anti-cancer therapy. Drug Resist Update. 2005;8:85–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

