Apoptotic effects of chrysin in human cancer cell lines
- PMID: 20559509
- PMCID: PMC2885101
- DOI: 10.3390/ijms11052188
Apoptotic effects of chrysin in human cancer cell lines
Abstract
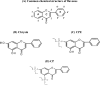
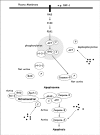
Chrysin is a natural flavonoid currently under investigation due to its important biological anti-cancer properties. In most of the cancer cells tested, chrysin has shown to inhibit proliferation and induce apoptosis, and is more potent than other tested flavonoids in leukemia cells, where chrysin is likely to act via activation of caspases and inactivation of Akt signaling in the cells. Moreover, structure-activity relationships have revealed that the chemical structure of chrysin meets the key structural requirements of flavonoids for potent cytotoxicity in leukemia cells. It is possible that combination therapy or modified chrysin could be more potent than single-agent use or administration of unmodified chrysin. This study may help to develop ways of improving the effectiveness of chrysin in the treatment of leukemia and other human cancers in vitro.
Keywords: apoptotic effect; chrysin; human cancers; in vitro.
Figures
Similar articles
-
Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells.Biochem Biophys Res Commun. 2004 Dec 24;325(4):1215-22. doi: 10.1016/j.bbrc.2004.09.225. Biochem Biophys Res Commun. 2004. PMID: 15555556
-
Synthesis and biological evaluation of amino acid derivatives containing chrysin that induce apoptosis.Nat Prod Res. 2021 Feb;35(4):529-538. doi: 10.1080/14786419.2019.1582043. Epub 2019 Mar 21. Nat Prod Res. 2021. PMID: 30897948
-
Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells.Anticancer Agents Med Chem. 2014;14(6):901-9. doi: 10.2174/1871520614666140209144042. Anticancer Agents Med Chem. 2014. PMID: 24521149
-
The Relationship between Pharmacological Properties and Structure- Activity of Chrysin Derivatives.Mini Rev Med Chem. 2019;19(7):555-568. doi: 10.2174/1389557518666180424094821. Mini Rev Med Chem. 2019. PMID: 29692242 Review.
-
Natural and Synthetic Flavonoids: Structure-Activity Relationship and Chemotherapeutic Potential for the Treatment of Leukemia.Crit Rev Food Sci Nutr. 2016 Jul 29;56 Suppl 1:S4-S28. doi: 10.1080/10408398.2015.1074532. Crit Rev Food Sci Nutr. 2016. PMID: 26463658 Review.
Cited by
-
Therapeutic potential of chrysin nanoparticle-mediation inhibition of succinate dehydrogenase and ubiquinone oxidoreductase in pancreatic and lung adenocarcinoma.Eur J Med Res. 2022 Sep 8;27(1):172. doi: 10.1186/s40001-022-00803-y. Eur J Med Res. 2022. PMID: 36076266 Free PMC article. Review.
-
Design and Development of Nanostructured Co Delivery of Artemisinin and Chrysin for Targeting hTERT Gene Expression in Breast Cancer Cell Line: Possible Clinical Application in Cancer Treatment.Asian Pac J Cancer Prev. 2022 Mar 1;23(3):919-927. doi: 10.31557/APJCP.2022.23.3.919. Asian Pac J Cancer Prev. 2022. PMID: 35345364 Free PMC article.
-
The Role of Epigenetic Modifications in Human Cancers and the Use of Natural Compounds as Epidrugs: Mechanistic Pathways and Pharmacodynamic Actions.Biomolecules. 2022 Feb 25;12(3):367. doi: 10.3390/biom12030367. Biomolecules. 2022. PMID: 35327559 Free PMC article. Review.
-
Improved In vivo Effect of Chrysin as an Absorption Enhancer Via the Preparation of Ternary Solid Dispersion with Brij®L4 and Aminoclay.Curr Drug Deliv. 2019;16(1):86-92. doi: 10.2174/1567201815666180924151458. Curr Drug Deliv. 2019. PMID: 30246640 Free PMC article.
-
Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review.Heliyon. 2020 Mar 7;6(3):e03557. doi: 10.1016/j.heliyon.2020.e03557. eCollection 2020 Mar. Heliyon. 2020. PMID: 32181408 Free PMC article. Review.
References
-
- Robards K, Antolovich M. Analytical chemistry of fruit bioflavonoids: A review. Analyst. 1997;122:11–34.
-
- Pietta PG. Flavonoids as anti-oxidants. J. Nat. Prod. 2000;63:1035–1042. - PubMed
-
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001;74:418–425. - PubMed
-
- Kale A, Gawande S, Kotwal S. Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytother. Res. 2008;22:567–577. - PubMed
-
- Awad R, Arnason JT, Trudeau V, Bergeron C, Budzinski JW, Foster BC, Merali Z. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties. Phytomedicine. 2003;10:640–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

