Inadequate clearance of translocated bacterial products in HIV-infected humanized mice
- PMID: 20442871
- PMCID: PMC2861703
- DOI: 10.1371/journal.ppat.1000867
Inadequate clearance of translocated bacterial products in HIV-infected humanized mice
Abstract
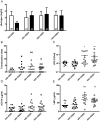
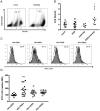
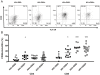
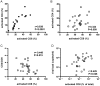
Bacterial translocation from the gut and subsequent immune activation are hallmarks of HIV infection and are thought to determine disease progression. Intestinal barrier integrity is impaired early in acute retroviral infection, but levels of plasma lipopolysaccharide (LPS), a marker of bacterial translocation, increase only later. We examined humanized mice infected with HIV to determine if disruption of the intestinal barrier alone is responsible for elevated levels of LPS and if bacterial translocation increases immune activation. Treating uninfected mice with dextran sodium sulfate (DSS) induced bacterial translocation, but did not result in elevated plasma LPS levels. DSS-induced translocation provoked LPS elevation only when phagocytic cells were depleted with clodronate liposomes (clodrolip). Macrophages of DSS-treated, HIV-negative mice phagocytosed more LPS ex vivo than those of control mice. In HIV-infected mice, however, LPS phagocytosis was insufficient to clear the translocated LPS. These conditions allowed higher levels of plasma LPS and CD8+ cell activation, which were associated with lower CD4+/CD8+ cell ratios and higher viral loads. LPS levels reflect both intestinal barrier and LPS clearance. Macrophages are essential in controlling systemic bacterial translocation, and this function might be hindered in chronic HIV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation.PLoS Pathog. 2013;9(6):e1003453. doi: 10.1371/journal.ppat.1003453. Epub 2013 Jun 20. PLoS Pathog. 2013. PMID: 23818854 Free PMC article. Clinical Trial.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Acupuncture for acute hordeolum.Cochrane Database Syst Rev. 2017 Feb 9;2(2):CD011075. doi: 10.1002/14651858.CD011075.pub2. Cochrane Database Syst Rev. 2017. PMID: 28181687 Free PMC article. Review.
Cited by
-
Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection.Nutrients. 2011 Dec;3(12):1042-70. doi: 10.3390/nu3121042. Epub 2011 Dec 19. Nutrients. 2011. PMID: 22292110 Free PMC article.
-
Complexities of Gut Microbiome Dysbiosis in the Context of HIV Infection and Antiretroviral Therapy.Clin Pharmacol Ther. 2016 Jun;99(6):600-11. doi: 10.1002/cpt.363. Epub 2016 Apr 16. Clin Pharmacol Ther. 2016. PMID: 26940481 Free PMC article. Review.
-
Host and Viral Factors in HIV-Mediated Bystander Apoptosis.Viruses. 2017 Aug 22;9(8):237. doi: 10.3390/v9080237. Viruses. 2017. PMID: 28829402 Free PMC article. Review.
-
Microbiota, natural products, and human health: exploring interactions for therapeutic insights.Front Cell Infect Microbiol. 2024 Jul 5;14:1371312. doi: 10.3389/fcimb.2024.1371312. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39035357 Free PMC article. Review.
-
Minocycline attenuates HIV-1 infection and suppresses chronic immune activation in humanized NOD/LtsZ-scidIL-2Rγ(null) mice.Immunology. 2014 Aug;142(4):562-72. doi: 10.1111/imm.12246. Immunology. 2014. PMID: 24409837 Free PMC article.
References
-
- Ancuta P, Kamat A, Kunstman KJ, Kim E-Y, Autissier P, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. - DOI - PMC - PubMed
-
- Gregson JN, Steel A, Bower M, Gazzard BG, Gotch FM, et al. Elevated plasma lipopolysaccharide is not sufficient to drive natural killer cell activation in HIV-1-infected individuals. AIDS. 2009;23:29–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

