Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome
- PMID: 20395444
- PMCID: PMC2877852
- DOI: 10.2353/ajpath.2010.091125
Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome
Abstract
Although Wnt/beta-catenin pathway activation has been implicated in mouse models of breast cancer, there is contradictory evidence regarding its importance in human breast cancer. In this study, invasive and in situ breast cancer tissue microarrays containing luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)(+)/ER(-) and basal-like breast cancers were analyzed for beta-catenin subcellular localization. We demonstrate that nuclear and cytosolic accumulation of beta-catenin, a read-out of Wnt pathway activation, was enriched in basal-like breast cancers. In contrast, membrane-associated beta-catenin was observed in all breast cancer subtypes, and its expression decreased with tumor progression. Moreover, nuclear and cytosolic localization of beta-catenin was associated with other markers of the basal-like phenotype, including nuclear hormone receptor and HER2 negativity, cytokeratin 5/6 and vimentin expression, and stem cell enrichment. Importantly, this subcellular localization of beta-catenin was associated with a poor outcome and is more frequently observed in tumors from black patients. In addition, beta-catenin accumulation was more often observed in basal-like in situ carcinomas than other in situ subtypes, suggesting that activation of this pathway might be an early event in basal-like tumor development. Collectively, these data indicate that Wnt/beta-catenin activation is an important feature of basal-like breast cancers and is predictive of worse overall survival, suggesting that it may be an attractive pharmacological target for this aggressive breast cancer subtype.
Figures
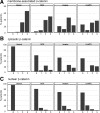
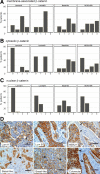

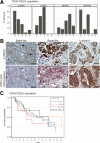
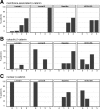
Similar articles
-
Activation status of Wnt/ß-catenin signaling in normal and neoplastic breast tissues: relationship to HER2/neu expression in human and mouse.PLoS One. 2012;7(3):e33421. doi: 10.1371/journal.pone.0033421. Epub 2012 Mar 23. PLoS One. 2012. PMID: 22457761 Free PMC article.
-
β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation.Mod Pathol. 2011 Feb;24(2):209-31. doi: 10.1038/modpathol.2010.205. Epub 2010 Nov 12. Mod Pathol. 2011. PMID: 21076461
-
Geldanamycin destabilizes HER2 tyrosine kinase and suppresses Wnt/beta-catenin signaling in HER2 overexpressing human breast cancer cells.Oncol Rep. 2007 Jan;17(1):89-96. Oncol Rep. 2007. PMID: 17143483
-
A Wnt-ow of opportunity: targeting the Wnt/beta-catenin pathway in breast cancer.Curr Drug Targets. 2010 Sep;11(9):1074-88. doi: 10.2174/138945010792006780. Curr Drug Targets. 2010. PMID: 20545611 Review.
-
Role of regulatory miRNAs of the Wnt/ β-catenin signaling pathway in tumorigenesis of breast cancer.Gene. 2020 Sep 5;754:144892. doi: 10.1016/j.gene.2020.144892. Epub 2020 Jun 10. Gene. 2020. PMID: 32534060 Review.
Cited by
-
Upregulated PFTK1 promotes tumor cell proliferation, migration, and invasion in breast cancer.Med Oncol. 2015 Jul;32(7):195. doi: 10.1007/s12032-015-0641-8. Epub 2015 Jun 2. Med Oncol. 2015. PMID: 26033031
-
FH535 inhibited migration and growth of breast cancer cells.PLoS One. 2012;7(9):e44418. doi: 10.1371/journal.pone.0044418. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984505 Free PMC article.
-
The Wnt Signalling Pathway: A Tailored Target in Cancer.Int J Mol Sci. 2020 Oct 18;21(20):7697. doi: 10.3390/ijms21207697. Int J Mol Sci. 2020. PMID: 33080952 Free PMC article. Review.
-
Activation status of Wnt/ß-catenin signaling in normal and neoplastic breast tissues: relationship to HER2/neu expression in human and mouse.PLoS One. 2012;7(3):e33421. doi: 10.1371/journal.pone.0033421. Epub 2012 Mar 23. PLoS One. 2012. PMID: 22457761 Free PMC article.
-
A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells.Oncogene. 2019 Apr;38(17):3151-3169. doi: 10.1038/s41388-018-0656-7. Epub 2019 Jan 8. Oncogene. 2019. PMID: 30622340 Free PMC article.
References
-
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. - PubMed
-
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8:145–158. - PubMed
-
- Zardawi SJ, O'Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog. Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24:385–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

