IL-7 is essential for homeostatic control of T cell metabolism in vivo
- PMID: 20194717
- PMCID: PMC2980949
- DOI: 10.4049/jimmunol.0902593
IL-7 is essential for homeostatic control of T cell metabolism in vivo
Abstract
It has become apparent that T cells require growth signals to maintain function and viability necessary to maintain proper immune homeostasis. One means by which cell extrinsic signals may mediate these effects is by sustaining sufficient basal cell metabolism to prevent cell atrophy. The role of metabolism and the specific growth factors essential to maintain metabolism of mature T cells in vivo, however, are poorly defined. As IL-7 is a nonredundant cytokine required for T cell development and survival and can regulate T cell metabolism in vitro, we hypothesized it may be essential to sustain metabolism of resting T cells in vivo. Thus, we generated a model for conditional expression of IL-7R in mature T cells. After IL-7R deletion in a generally normal lymphoid environment, T cells had reduced responses to IL-7, including abrogated signaling and maintenance of antiapoptotic Bcl-2 family expression that corresponded to decreased survival in vitro. T cell survival in vivo was also reduced after loss of the IL-7R in a T cell-intrinsic manner. Additionally, IL-7R deletion resulted in delayed growth and proliferation following stimulation. Importantly, in vivo excision of IL-7R led to T cell atrophy that was characterized by delayed mitogenesis and reduced glycolytic flux. These data are the first to identify an in vivo requirement for a specific cell extrinsic signal to sustain lymphocyte metabolism and suggest that control of glycolysis by IL-7R may contribute to the well-described roles of IL-7 in T cell development, homeostatic proliferation, and survival.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

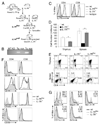
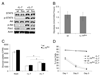
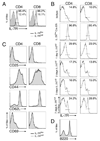

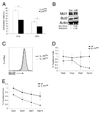
Similar articles
-
IL-7R-Dependent Phosphatidylinositol 3-Kinase Competes with the STAT5 Signal to Modulate T Cell Development and Homeostasis.J Immunol. 2020 Feb 15;204(4):844-857. doi: 10.4049/jimmunol.1900456. Epub 2020 Jan 10. J Immunol. 2020. PMID: 31924648
-
Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells.Eur J Immunol. 2007 Nov;37(11):3078-88. doi: 10.1002/eji.200737585. Eur J Immunol. 2007. PMID: 17935075
-
Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naïve CD8(+) T cells.Immunol Cell Biol. 2011 Jul;89(5):581-94. doi: 10.1038/icb.2011.5. Epub 2011 Feb 22. Immunol Cell Biol. 2011. PMID: 21339767 Free PMC article.
-
Flip the coin: IL-7 and IL-7R in health and disease.Nat Immunol. 2019 Dec;20(12):1584-1593. doi: 10.1038/s41590-019-0479-x. Epub 2019 Nov 19. Nat Immunol. 2019. PMID: 31745336 Review.
-
Mucosal T cells as a target for treatment of IBD.J Gastroenterol. 2003 Mar;38 Suppl 15:48-50. J Gastroenterol. 2003. PMID: 12698871 Review.
Cited by
-
Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response.Nat Commun. 2011 Dec 6;2:576. doi: 10.1038/ncomms1585. Nat Commun. 2011. PMID: 22146395 Free PMC article.
-
Differential Cytokine Utilization and Tissue Tropism Results in Distinct Repopulation Kinetics of Naïve vs. Memory T Cells in Mice.Front Immunol. 2019 Mar 4;10:355. doi: 10.3389/fimmu.2019.00355. eCollection 2019. Front Immunol. 2019. PMID: 30886618 Free PMC article.
-
IL-33 drives the production of mouse regulatory T cells with enhanced in vivo suppressive activity in skin transplantation.Am J Transplant. 2021 Mar;21(3):978-992. doi: 10.1111/ajt.16266. Epub 2020 Sep 15. Am J Transplant. 2021. PMID: 33314772 Free PMC article.
-
Mitochondrial dynamics and metabolic regulation control T cell fate in the thymus.Front Immunol. 2024 Jan 15;14:1270268. doi: 10.3389/fimmu.2023.1270268. eCollection 2023. Front Immunol. 2024. PMID: 38288115 Free PMC article.
-
Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis.Oncogene. 2011 Apr 21;30(16):1855-67. doi: 10.1038/onc.2010.561. Epub 2010 Dec 13. Oncogene. 2011. PMID: 21151168 Free PMC article.
References
-
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. - PubMed
-
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell. 2000;6:683–692. - PubMed
-
- Plas DR, Rathmell JC, Thompson CB. Homeostatic control of lymphocyte survival: potential origins and implications. Nat. Immunol. 2002;3:515–521. - PubMed
-
- Edinger AL, Cinalli RM, Thompson CB. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev. Cell. 2003;5:571–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

