Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress
- PMID: 20126446
- PMCID: PMC2813276
- DOI: 10.1371/journal.ppat.1000742
Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress
Abstract
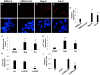
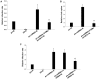
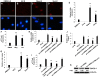
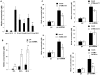
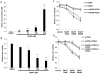
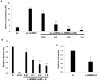
Upregulation of xCT, the inducible subunit of a membrane-bound amino acid transporter, replenishes intracellular glutathione stores to maintain cell viability in an environment of oxidative stress. xCT also serves as a fusion-entry receptor for the Kaposi's sarcoma-associated herpesvirus (KSHV), the causative agent of Kaposi's sarcoma (KS). Ongoing KSHV replication and infection of new cell targets is important for KS progression, but whether xCT regulation within the tumor microenvironment plays a role in KS pathogenesis has not been determined. Using gene transfer and whole virus infection experiments, we found that KSHV-encoded microRNAs (KSHV miRNAs) upregulate xCT expression by macrophages and endothelial cells, largely through miR-K12-11 suppression of BACH-1-a negative regulator of transcription recognizing antioxidant response elements within gene promoters. Correlative functional studies reveal that upregulation of xCT by KSHV miRNAs increases cell permissiveness for KSHV infection and protects infected cells from death induced by reactive nitrogen species (RNS). Interestingly, KSHV miRNAs simultaneously upregulate macrophage secretion of RNS, and biochemical inhibition of RNS secretion by macrophages significantly reduces their permissiveness for KSHV infection. The clinical relevance of these findings is supported by our demonstration of increased xCT expression within more advanced human KS tumors containing a larger number of KSHV-infected cells. Collectively, these data support a role for KSHV itself in promoting de novo KSHV infection and the survival of KSHV-infected, RNS-secreting cells in the tumor microenvironment through the induction of xCT.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Kaposi's sarcoma-associated herpesvirus suppression of DUSP1 facilitates cellular pathogenesis following de novo infection.J Virol. 2013 Jan;87(1):621-35. doi: 10.1128/JVI.01441-12. Epub 2012 Oct 24. J Virol. 2013. PMID: 23097457 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
Modified Cross-Linking, Ligation, and Sequencing of Hybrids (qCLASH) Identifies Kaposi's Sarcoma-Associated Herpesvirus MicroRNA Targets in Endothelial Cells.J Virol. 2018 Mar 28;92(8):e02138-17. doi: 10.1128/JVI.02138-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29386283 Free PMC article.
-
KSHV microRNAs: Tricks of the Devil.Trends Microbiol. 2017 Aug;25(8):648-661. doi: 10.1016/j.tim.2017.02.002. Epub 2017 Mar 2. Trends Microbiol. 2017. PMID: 28259385 Free PMC article. Review.
-
miRNAs and their roles in KSHV pathogenesis.Virus Res. 2019 Jun;266:15-24. doi: 10.1016/j.virusres.2019.03.024. Epub 2019 Apr 2. Virus Res. 2019. PMID: 30951791 Review.
Cited by
-
Looking at Kaposi's Sarcoma-Associated Herpesvirus-Host Interactions from a microRNA Viewpoint.Front Microbiol. 2012 Jan 11;2:271. doi: 10.3389/fmicb.2011.00271. eCollection 2011. Front Microbiol. 2012. PMID: 22275910 Free PMC article.
-
Potential pitfalls in microRNA profiling.Wiley Interdiscip Rev RNA. 2012 Sep-Oct;3(5):601-16. doi: 10.1002/wrna.1120. Epub 2012 May 7. Wiley Interdiscip Rev RNA. 2012. PMID: 22566380 Free PMC article. Review.
-
Functions of Kaposi's sarcoma-associated herpesvirus microRNAs.Biochim Biophys Acta. 2011 Nov-Dec;1809(11-12):623-30. doi: 10.1016/j.bbagrm.2011.05.003. Epub 2011 May 18. Biochim Biophys Acta. 2011. PMID: 21616184 Free PMC article. Review.
-
Apoptosis - an Ubiquitous T cell Immunomodulator.J Clin Cell Immunol. 2011 Dec 10;S3:2. doi: 10.4172/2155-9899.S3-002. J Clin Cell Immunol. 2011. PMID: 23504027 Free PMC article.
-
HPV16 integration regulates ferroptosis resistance via the c-Myc/miR-142-5p/HOXA5/SLC7A11 axis during cervical carcinogenesis.Cell Biosci. 2024 Oct 17;14(1):129. doi: 10.1186/s13578-024-01309-2. Cell Biosci. 2024. PMID: 39420439 Free PMC article.
References
-
- Luppi M, Barozzi P, Schulz TF, Setti G, Staskus K, et al. Bone marrow failure associated with human herpesvirus 8 infection after transplantation. N Engl J Med. 2000;343:1378–1385. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

