Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells
- PMID: 20093776
- PMCID: PMC2810085
- DOI: 10.1172/JCI40483
Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells
Abstract
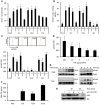
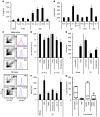
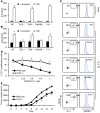
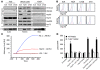
Myeloid-derived suppressor cells (MDSCs) have been identified in humans and mice as a population of immature myeloid cells with the ability to suppress T cell activation. They accumulate in tumor-bearing mice and humans and have been shown to contribute to cancer development. Here, we have isolated tumor-derived exosomes (TDEs) from mouse cell lines and shown that an interaction between TDE-associated Hsp72 and MDSCs determines the suppressive activity of the MDSCs via activation of Stat3. In addition, tumor-derived soluble factors triggered MDSC expansion via activation of Erk. TDE-associated Hsp72 triggered Stat3 activation in MDSCs in a TLR2/MyD88-dependent manner through autocrine production of IL-6. Importantly, decreasing exosome production using dimethyl amiloride enhanced the in vivo antitumor efficacy of the chemotherapeutic drug cyclophosphamide in 3 different mouse tumor models. We also demonstrated that this mechanism is relevant in cancer patients, as TDEs from a human tumor cell line activated human MDSCs and triggered their suppressive function in an Hsp72/TLR2-dependent manner. Further, MDSCs from cancer patients treated with amiloride, a drug used to treat high blood pressure that also inhibits exosome formation, exhibited reduced suppressor functions. Collectively, our findings show in both mice and humans that Hsp72 expressed at the surface of TDEs restrains tumor immune surveillance by promoting MDSC suppressive functions.
Figures

Similar articles
-
Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression.Cell Commun Signal. 2020 Jul 8;18(1):106. doi: 10.1186/s12964-020-00611-z. Cell Commun Signal. 2020. PMID: 32641056 Free PMC article.
-
Repression of MUC1 Promotes Expansion and Suppressive Function of Myeloid-Derived Suppressor Cells in Pancreatic and Breast Cancer Murine Models.Int J Mol Sci. 2021 May 25;22(11):5587. doi: 10.3390/ijms22115587. Int J Mol Sci. 2021. PMID: 34070449 Free PMC article.
-
Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3.Med Oncol. 2015 Feb;32(2):453. doi: 10.1007/s12032-014-0453-2. Epub 2015 Jan 21. Med Oncol. 2015. PMID: 25603952
-
Suppression of T cells by myeloid-derived suppressor cells in cancer.Hum Immunol. 2017 Feb;78(2):113-119. doi: 10.1016/j.humimm.2016.12.001. Epub 2016 Dec 7. Hum Immunol. 2017. PMID: 27939507 Review.
-
Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment.Adv Cancer Res. 2015;128:95-139. doi: 10.1016/bs.acr.2015.04.002. Epub 2015 May 12. Adv Cancer Res. 2015. PMID: 26216631 Free PMC article. Review.
Cited by
-
Extracellular vesicles in liver pathobiology: Small particles with big impact.Hepatology. 2016 Dec;64(6):2219-2233. doi: 10.1002/hep.28814. Epub 2016 Oct 20. Hepatology. 2016. PMID: 27628960 Free PMC article. Review.
-
Exosomes in the Pathogenesis, Diagnosis and Treatment of Pancreatic Diseases.CellR4 Repair Replace Regen Reprogram. 2014;2(1):e807. Epub 2014 Feb 8. CellR4 Repair Replace Regen Reprogram. 2014. PMID: 33869660 Free PMC article.
-
Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation.Front Immunol. 2020 Jul 22;11:1371. doi: 10.3389/fimmu.2020.01371. eCollection 2020. Front Immunol. 2020. PMID: 32793192 Free PMC article. Review.
-
Exosomes in cancer: small particle, big player.J Hematol Oncol. 2015 Jul 10;8:83. doi: 10.1186/s13045-015-0181-x. J Hematol Oncol. 2015. PMID: 26156517 Free PMC article. Review.
-
STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia.Mucosal Immunol. 2013 Jan;6(1):189-99. doi: 10.1038/mi.2012.62. Epub 2012 Jul 11. Mucosal Immunol. 2013. PMID: 22785228 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

