Probiotics promote gut health through stimulation of epithelial innate immunity
- PMID: 20018654
- PMCID: PMC2806692
- DOI: 10.1073/pnas.0910307107
Probiotics promote gut health through stimulation of epithelial innate immunity
Abstract
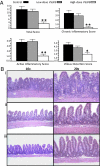
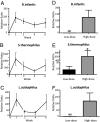
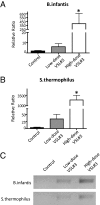
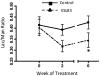
Probiotic formulations are widely available and have a variety of proposed beneficial effects, including promotion of gut health. The mechanisms of action of probiotic bacteria in the intestine are still unclear but are generally attributed to an antiinflammatory effect. Here, we demonstrate that the multiple probiotic formulation VSL#3 prevents the onset of intestinal inflammation by local stimulation of epithelial innate immune responses (i.e., increased production of epithelial-derived TNF-alpha and restoration of epithelial barrier function in vivo). We also demonstrate that probiotic bacteria stimulate epithelial production of TNF-alpha and activate NF-kappaB in vitro. Our results support the hypothesis that probiotics promote gut health through stimulation, rather than suppression, of the innate immune system. Furthermore, our findings provide the perspective that defects in innate immunity may play a critical role in the pathogenesis and progression of intestinal disorders, such as inflammatory bowel disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
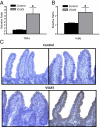
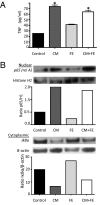
Similar articles
-
Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism.PLoS One. 2012;7(7):e42067. doi: 10.1371/journal.pone.0042067. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848704 Free PMC article.
-
Probiotics and ileitis: could augmentation of TNF/NFκB activity be the answer?Gut Microbes. 2010 May-Jun;1(3):196-9. doi: 10.4161/gmic.1.3.12485. Epub 2010 May 26. Gut Microbes. 2010. PMID: 21327025 Free PMC article.
-
Unraveling mechanisms of action of probiotics.Nutr Clin Pract. 2009 Feb-Mar;24(1):10-4. doi: 10.1177/0884533608329231. Nutr Clin Pract. 2009. PMID: 19244144 Review.
-
Influences of enteral nutrition combined with probiotics on gut microflora and barrier function of rats with abdominal infection.World J Gastroenterol. 2006 Jul 21;12(27):4352-8. doi: 10.3748/wjg.v12.i27.4352. World J Gastroenterol. 2006. PMID: 16865777 Free PMC article.
-
Role of endogenous microbiota, probiotics and their biological products in human health.Nutrients. 2013 Jan 10;5(1):58-81. doi: 10.3390/nu5010058. Nutrients. 2013. PMID: 23306189 Free PMC article. Review.
Cited by
-
Comparative evaluation of fermented ginseng on alleviating antibiotic-associated diarrhea in mice.Food Sci Biotechnol. 2024 May 11;33(12):2845-2856. doi: 10.1007/s10068-024-01538-8. eCollection 2024 Sep. Food Sci Biotechnol. 2024. PMID: 39184984
-
Examining immune-inflammatory mechanisms of probiotic supplementation in depression: secondary findings from a randomized clinical trial.Transl Psychiatry. 2024 Jul 24;14(1):305. doi: 10.1038/s41398-024-03030-7. Transl Psychiatry. 2024. PMID: 39048549 Free PMC article. Clinical Trial.
-
The Viable but Non-Culturable (VBNC) State, a Poorly Explored Aspect of Beneficial Bacteria.Microorganisms. 2023 Dec 25;12(1):39. doi: 10.3390/microorganisms12010039. Microorganisms. 2023. PMID: 38257865 Free PMC article. Review.
-
Effects of Lactobacillus plantarum and Weissella viridescens on the Gut Microbiota and Serum Metabolites of Mice with Antibiotic-Associated Diarrhea.Nutrients. 2023 Oct 30;15(21):4603. doi: 10.3390/nu15214603. Nutrients. 2023. PMID: 37960257 Free PMC article.
-
Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease.Intest Res. 2024 Jan;22(1):15-43. doi: 10.5217/ir.2023.00080. Epub 2023 Nov 8. Intest Res. 2024. PMID: 37935653 Free PMC article. Review.
References
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. - PubMed
-
- Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Bamias G, Nyce MR, De La Rue SA, Cominelli F American College of Physicians; American Physiological Society. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. - PubMed
-
- Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev. 2005;206:277–295. - PubMed
-
- Marks DJ, et al. Defective acute inflammation in Crohn’s disease: A clinical investigation. Lancet. 2006;367:668–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

