Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia
- PMID: 20008620
- PMCID: PMC2815702
- DOI: 10.1200/JCO.2009.25.4920
Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia
Abstract
Purpose: Dasatinib is effective therapy for chronic myeloid leukemia (CML) after imatinib failure. In this study, we investigate the efficacy of dasatinib as initial therapy for patients with CML in early chronic phase.
Patients and methods: Patients with newly diagnosed CML in early chronic phase were randomly assigned to receive dasatinib 100 mg once daily or 50 mg twice daily as initial therapy.
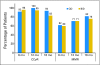
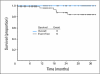
Results: Among 50 patients observed for at least 3 months, 49 patients (98%) achieved a complete cytogenetic response (CCyR), and 41 patients (82%) achieved a major molecular response (MMR). Responses occurred rapidly, with 94% of patients achieving CCyR by 6 months. There was no difference in response rate by treatment arm. The projected event-free survival rate at 24 months is 88%, and all patients are alive after a median follow-up time of 24 months. Grade >or= 3 neutropenia and thrombocytopenia occurred in 21% and 10% of patients, respectively. Nonhematologic toxicity was usually grade 1 to 2. There was no significant difference in toxicity between the two arms, and the actual median dose at 12 months was 100 mg (range, 20 to 100 mg).
Conclusion: Dasatinib is an effective agent for the initial management of CML in early chronic phase, producing high rates of CCyR and MMR.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Chronic myeloid leukemia: reversing the chronic phase.J Clin Oncol. 2010 Jan 20;28(3):363-5. doi: 10.1200/JCO.2009.26.5587. Epub 2009 Dec 14. J Clin Oncol. 2010. PMID: 20008612 No abstract available.
Similar articles
-
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia.N Engl J Med. 2010 Jun 17;362(24):2260-70. doi: 10.1056/NEJMoa1002315. Epub 2010 Jun 5. N Engl J Med. 2010. PMID: 20525995 Clinical Trial.
-
Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION).Blood. 2012 Feb 2;119(5):1123-9. doi: 10.1182/blood-2011-08-376087. Epub 2011 Dec 9. Blood. 2012. PMID: 22160483 Free PMC article. Clinical Trial.
-
[Preliminary comparison of efficacy and safety of dasatinib and imatinib in newly diagnosed chronic myeloid leukemia].Zhonghua Xue Ye Xue Za Zhi. 2013 Feb;34(2):93-7. Zhonghua Xue Ye Xue Za Zhi. 2013. PMID: 23611212 Clinical Trial. Chinese.
-
Dasatinib: from treatment of imatinib-resistant or -intolerant patients with chronic myeloid leukemia to treatment of patients with newly diagnosed chronic phase chronic myeloid leukemia.Clin Ther. 2012 Feb;34(2):272-81. doi: 10.1016/j.clinthera.2012.01.009. Epub 2012 Jan 27. Clin Ther. 2012. PMID: 22285209 Review.
-
Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia: systematic reviews and economic analyses.Health Technol Assess. 2012;16(42):iii-iv, 1-277. doi: 10.3310/hta16420. Health Technol Assess. 2012. PMID: 23134589 Review.
Cited by
-
Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors.Am J Hematol. 2013 Dec;88(12):1024-9. doi: 10.1002/ajh.23560. Epub 2013 Sep 12. Am J Hematol. 2013. PMID: 23913852 Free PMC article.
-
Chronic myeloid leukemia: In pursuit of perfection.South Asian J Cancer. 2012 Jul;1(1):16-24. doi: 10.4103/2278-330X.96498. South Asian J Cancer. 2012. PMID: 24455503 Free PMC article.
-
Outcome of treatment of chronic myeloid leukemia with second-generation tyrosine kinase inhibitors after imatinib failure.Clin Lymphoma Myeloma Leuk. 2011 Jun;11 Suppl 1(0 1):S101-10. doi: 10.1016/j.clml.2011.02.009. Epub 2011 Apr 28. Clin Lymphoma Myeloma Leuk. 2011. PMID: 22035738 Free PMC article.
-
Malignancies occurring during therapy with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) and other hematologic malignancies.Blood. 2011 Oct 20;118(16):4353-8. doi: 10.1182/blood-2011-06-362889. Epub 2011 Aug 16. Blood. 2011. PMID: 21846902 Free PMC article.
-
Reversible ventricular arrythmia induced by dasatinib.Clin Case Rep. 2013 Oct;1(1):20-5. doi: 10.1002/ccr3.5. Epub 2013 Jul 31. Clin Case Rep. 2013. PMID: 25356203 Free PMC article.
References
-
- O'Brien SG, Guilhot F, Goldman J, et al. International Randomized Study of Interferon Versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (IM) Blood. 2008;112:186. abstr.
-
- Kantarjian HM, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. - PubMed
-
- Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: Historical comparison between two phase 3 trials. Blood. 2006;108:1478–1484. - PubMed
-
- Baccarani M, Martinelli G, Rosti G, et al. Imatinib and pegylated human recombinant interferon-alpha2b in early chronic-phase chronic myeloid leukemia. Blood. 2004;104:4245–4251. - PubMed
-
- Kantarjian H, Talpaz M, O'Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

