Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells
- PMID: 19923183
- PMCID: PMC2820927
- DOI: 10.1128/JVI.01334-09
Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells
Abstract
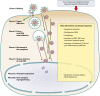
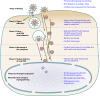
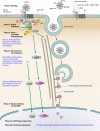
Kaposi's sarcoma-associated herpesvirus (KSHV), the most recently identified member of the herpesvirus family, infects a variety of target cells in vitro and in vivo. This minireview surveys current information on the early events of KSHV infection, including virus-receptor interactions, involved envelope glycoproteins, mode of entry, intracellular trafficking, and initial viral and host gene expression programs. We describe data supporting the hypothesis that KSHV manipulates preexisting host cell signaling pathways to allow successful infection. The various signaling events triggered by infection, and their potential roles in the different stages of infection and disease pathogenesis, are summarized.
Figures
Similar articles
-
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells.J Virol. 2015 Mar;89(6):3093-111. doi: 10.1128/JVI.02507-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552714 Free PMC article.
-
Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry.J Virol. 2007 Aug;81(15):7941-59. doi: 10.1128/JVI.02848-06. Epub 2007 May 16. J Virol. 2007. PMID: 17507466 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection.J Virol. 2008 Dec;82(24):12126-44. doi: 10.1128/JVI.01146-08. Epub 2008 Oct 1. J Virol. 2008. PMID: 18829766 Free PMC article.
-
Insight into the Roles of E3 Ubiquitin Ligase c-Cbl, ESCRT Machinery, and Host Cell Signaling in Kaposi's Sarcoma-Associated Herpesvirus Entry and Trafficking.J Virol. 2018 Jan 30;92(4):e01376-17. doi: 10.1128/JVI.01376-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29167336 Free PMC article. Review.
-
KSHV Entry and Trafficking in Target Cells-Hijacking of Cell Signal Pathways, Actin and Membrane Dynamics.Viruses. 2016 Nov 14;8(11):305. doi: 10.3390/v8110305. Viruses. 2016. PMID: 27854239 Free PMC article. Review.
Cited by
-
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells.J Virol. 2015 Mar;89(6):3093-111. doi: 10.1128/JVI.02507-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552714 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes.J Virol. 2013 Apr;87(8):4417-31. doi: 10.1128/JVI.03282-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388709 Free PMC article.
-
Herpesviruses and intermediate filaments: close encounters with the third type.Viruses. 2011 Jul;3(7):1015-40. doi: 10.3390/v3071015. Epub 2011 Jul 4. Viruses. 2011. PMID: 21994768 Free PMC article. Review.
-
Evasion and subversion of interferon-mediated antiviral immunity by Kaposi's sarcoma-associated herpesvirus: an overview.J Virol. 2011 Nov;85(21):10934-44. doi: 10.1128/JVI.00687-11. Epub 2011 Jul 20. J Virol. 2011. PMID: 21775463 Free PMC article. Review.
-
Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line.J Exp Clin Cancer Res. 2013 Oct 23;32(1):79. doi: 10.1186/1756-9966-32-79. J Exp Clin Cancer Res. 2013. PMID: 24422998 Free PMC article.
References
-
- Akula, S. M., P. P. Naranatt, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. - PubMed
-
- Akula, S. M., P. P. Naranatt, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8) entry into target cells. Cell 108:407-419. - PubMed
-
- Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

