GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis
- PMID: 19917257
- PMCID: PMC2782904
- DOI: 10.1016/j.molcel.2009.10.021
GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis
Abstract
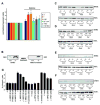
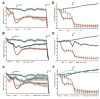
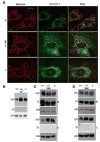
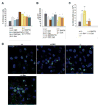
Mitochondria-associated ER membranes, or MAMs, define the sites of endoplasmic reticulum/mitochondria juxtaposition that control Ca(2+) flux between these organelles. We found that in a mouse model of the human lysosomal storage disease GM1-gangliosidosis, GM1-ganglioside accumulates in the glycosphingolipid-enriched microdomain (GEM) fractions of MAMs, where it interacts with the phosphorylated form of IP3 receptor-1, influencing the activity of this channel. Ca(2+) depleted from the ER is then taken up by the mitochondria, leading to Ca(2+) overload in this organelle. The latter induces mitochondrial membrane permeabilization (MMP), opening of the permeability transition pore, and activation of the mitochondrial apoptotic pathway. This study identifies the GEMs as the sites of Ca(2+) diffusion between the ER and the mitochondria. We propose a new mechanism of Ca(2+)-mediated apoptotic signaling whereby GM1 accumulation at the GEMs alters Ca(2+) dynamics and acts as a molecular effector of both ER stress-induced and mitochondria-mediated apoptosis of neuronal cells.
Figures



Similar articles
-
Altered GM1 catabolism affects NMDAR-mediated Ca2+ signaling at ER-PM junctions and increases synaptic spine formation in a GM1-gangliosidosis model.Cell Rep. 2024 May 28;43(5):114117. doi: 10.1016/j.celrep.2024.114117. Epub 2024 Apr 16. Cell Rep. 2024. PMID: 38630590 Free PMC article.
-
Mitochondria-associated ER membranes (MAMs) and glycosphingolipid enriched microdomains (GEMs): isolation from mouse brain.J Vis Exp. 2013 Mar 4;(73):e50215. doi: 10.3791/50215. J Vis Exp. 2013. PMID: 23486347 Free PMC article.
-
GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis.Mol Cell. 2004 Sep 10;15(5):753-66. doi: 10.1016/j.molcel.2004.08.029. Mol Cell. 2004. PMID: 15350219
-
Endoplasmic Reticulum-Mitochondria Communication Through Ca2+ Signaling: The Importance of Mitochondria-Associated Membranes (MAMs).Adv Exp Med Biol. 2017;997:49-67. doi: 10.1007/978-981-10-4567-7_4. Adv Exp Med Biol. 2017. PMID: 28815521 Review.
-
The ER-mitochondria interface, where Ca2+ and cell death meet.Cell Calcium. 2023 Jun;112:102743. doi: 10.1016/j.ceca.2023.102743. Epub 2023 Apr 25. Cell Calcium. 2023. PMID: 37126911 Review.
Cited by
-
Secondary Mitochondrial Dysfunction as a Cause of Neurodegenerative Dysfunction in Lysosomal Storage Diseases and an Overview of Potential Therapies.Int J Mol Sci. 2022 Sep 12;23(18):10573. doi: 10.3390/ijms231810573. Int J Mol Sci. 2022. PMID: 36142486 Free PMC article. Review.
-
Isolation of Mitochondria-Associated ER Membranes (MAMs), Synaptic MAMs, and Glycosphingolipid Enriched Microdomains (GEMs) from Brain Tissues and Neuronal Cells.Methods Mol Biol. 2021;2277:357-370. doi: 10.1007/978-1-0716-1270-5_22. Methods Mol Biol. 2021. PMID: 34080162
-
The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction.J Cell Biol. 2012 Nov 26;199(5):723-34. doi: 10.1083/jcb.201208152. J Cell Biol. 2012. PMID: 23185029 Free PMC article. Review.
-
Elevation of GM2 ganglioside during ethanol-induced apoptotic neurodegeneration in the developing mouse brain.J Neurochem. 2012 May;121(4):649-61. doi: 10.1111/j.1471-4159.2012.07710.x. Epub 2012 Mar 20. J Neurochem. 2012. PMID: 22372857 Free PMC article.
-
Dysregulation of cellular membrane homeostasis as a crucial modulator of cancer risk.FEBS J. 2024 Apr;291(7):1299-1352. doi: 10.1111/febs.16665. Epub 2022 Nov 7. FEBS J. 2024. PMID: 36282100 Review.
References
-
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. - PubMed
-
- Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J Biol Chem. 2006;281:17347–17358. - PubMed
-
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. - PubMed
-
- Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci. 2001;26:112–117. - PubMed
-
- d'Azzo A, Tessitore A, Sano R. Gangliosides as apoptotic signals in ER stress response. Cell Death Differ. 2006;13:404–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

