Molecular and clinical assessment in the treatment of AIDS Kaposi sarcoma with valproic Acid
- PMID: 19911999
- PMCID: PMC2952388
- DOI: 10.1086/648447
Molecular and clinical assessment in the treatment of AIDS Kaposi sarcoma with valproic Acid
Abstract
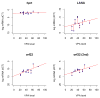
The AIDS Malignancy Consortium undertook a pilot trial of valproic acid among patients with AIDS-associated Kaposi sarcoma (KS). Treatment was associated with low toxicity, but the KS clinical response and KS herpesvirus lytic induction rates were not sufficiently high to meet predefined criteria for efficacy.
Conflict of interest statement
All authors: no conflicts
Figures

Similar articles
-
Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe.Clin Infect Dis. 2010 Aug 1;51(3):342-9. doi: 10.1086/654800. Clin Infect Dis. 2010. PMID: 20572760
-
The influence of highly active antiretroviral therapy on AIDS-associated Kaposi's sarcoma.Br J Dermatol. 1999 May;140(5):875-81. doi: 10.1046/j.1365-2133.1999.02818.x. Br J Dermatol. 1999. PMID: 10354025
-
Acquired immunodeficiency syndrome-related Kaposi's sarcoma regression after highly active antiretroviral therapy: biologic correlates of clinical outcome.J Natl Cancer Inst Monogr. 2001;(28):44-9. doi: 10.1093/oxfordjournals.jncimonographs.a024256. J Natl Cancer Inst Monogr. 2001. PMID: 11158206
-
Treatment of AIDS-related Kaposi's sarcoma.Hematol Oncol Clin North Am. 1991 Apr;5(2):297-310. Hematol Oncol Clin North Am. 1991. PMID: 2022595 Review.
-
Kaposi's sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology.Am J Epidemiol. 1998 Feb 1;147(3):217-21. doi: 10.1093/oxfordjournals.aje.a009440. Am J Epidemiol. 1998. PMID: 9482495 Review. No abstract available.
Cited by
-
Chromatin remodeling controls Kaposi's sarcoma-associated herpesvirus reactivation from latency.PLoS Pathog. 2018 Sep 13;14(9):e1007267. doi: 10.1371/journal.ppat.1007267. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30212584 Free PMC article.
-
Restricted Kaposi's sarcoma (KS) herpesvirus transcription in KS lesions from patients on successful antiretroviral therapy.mBio. 2011 Nov 1;2(6):e00138-11. doi: 10.1128/mBio.00138-11. Print 2011. mBio. 2011. PMID: 22045987 Free PMC article.
-
Pilot Trial AMC-063: Safety and Efficacy of Bortezomib in AIDS-associated Kaposi Sarcoma.Clin Cancer Res. 2020 Feb 1;26(3):558-565. doi: 10.1158/1078-0432.CCR-19-1044. Epub 2019 Oct 17. Clin Cancer Res. 2020. PMID: 31624104 Free PMC article. Clinical Trial.
-
Bortezomib-induced Epstein-Barr virus and Kaposi sarcoma herpesvirus lytic gene expression: oncolytic strategies.Curr Opin Oncol. 2011 Sep;23(5):482-7. doi: 10.1097/CCO.0b013e3283499c37. Curr Opin Oncol. 2011. PMID: 21788895 Free PMC article. Review.
-
Valproic acid antagonizes the capacity of other histone deacetylase inhibitors to activate the Epstein-barr virus lytic cycle.J Virol. 2011 Jun;85(11):5628-43. doi: 10.1128/JVI.02659-10. Epub 2011 Mar 16. J Virol. 2011. PMID: 21411522 Free PMC article.
References
-
- Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007 Sep 27;357(13):1352–3. - PubMed
-
- Ambinder RF, Cesarman E. Clinical and pathological aspects of EBV and KSHV infection. In: Arvin A, Campadelli-Fiume G, Moore PS, editors. Human Herpesviruses Biology, Therapy, and Immunoprophylaxis. New York: Cambridge University Press; 2007. pp. 885–914. - PubMed
-
- Shaw RN, Arbiser JL, Offermann MK. Valproic acid induces human herpesvirus 8 lytic gene expression in BCBL-1 cells. AIDS. 2000 May 5;14(7):899–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01CA070062/CA/NCI NIH HHS/United States
- DE018304/DE/NIDCR NIH HHS/United States
- U01CA70054/CA/NCI NIH HHS/United States
- U01CA070019/CA/NCI NIH HHS/United States
- R01 DE018304-02/DE/NIDCR NIH HHS/United States
- U01 CA070019-09/CA/NCI NIH HHS/United States
- U01 CA070062/CA/NCI NIH HHS/United States
- U01 CA121947/CA/NCI NIH HHS/United States
- R01 CA109232/CA/NCI NIH HHS/United States
- R01 CA163217/CA/NCI NIH HHS/United States
- U01 CA070047/CA/NCI NIH HHS/United States
- U01 CA070019-08/CA/NCI NIH HHS/United States
- U01 CA070019/CA/NCI NIH HHS/United States
- R01 DE018304-03/DE/NIDCR NIH HHS/United States
- U01 CA083035/CA/NCI NIH HHS/United States
- P01 CA081400-04/CA/NCI NIH HHS/United States
- P01 CA113239/CA/NCI NIH HHS/United States
- U01 CA066535/CA/NCI NIH HHS/United States
- U01 CA083118/CA/NCI NIH HHS/United States
- U01CA121947/CA/NCI NIH HHS/United States
- U01 CA071375/CA/NCI NIH HHS/United States
- R01 DE018304/DE/NIDCR NIH HHS/United States
- U01CA083118/CA/NCI NIH HHS/United States
- U01CA071375/CA/NCI NIH HHS/United States
- U01CA070047/CA/NCI NIH HHS/United States
- U01 CA070054/CA/NCI NIH HHS/United States
- P01CA113239/CA/NCI NIH HHS/United States
- UO1CA66535/CA/NCI NIH HHS/United States
- CA109232/CA/NCI NIH HHS/United States
- U01 CA121947-016805/CA/NCI NIH HHS/United States
- R01 DE018304-01/DE/NIDCR NIH HHS/United States
- U01CA083035/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

