Tumor necrosis factor alpha represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB
- PMID: 19854828
- PMCID: PMC2791026
- DOI: 10.1074/jbc.M109.070540
Tumor necrosis factor alpha represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB
Abstract
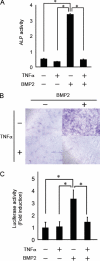
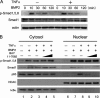
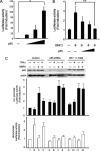
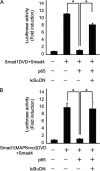
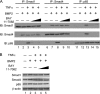
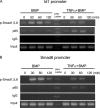
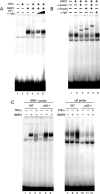
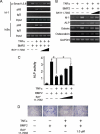
Bone morphogenetic proteins (BMPs) induce not only bone formation in vivo but also osteoblast differentiation of mesenchymal cells in vitro. Tumor necrosis factor alpha (TNFalpha) inhibits both osteoblast differentiation and bone formation induced by BMPs. However, the molecular mechanisms of these inhibitions remain unknown. In this study, we found that TNFalpha inhibited the alkaline phosphatase activity and markedly reduced BMP2- and Smad-induced reporter activity in MC3T3-E1 cells. TNFalpha had no effect on the phosphorylation of Smad1, Smad5, and Smad8 or on the nuclear translocation of the Smad1-Smad4 complex. In p65-deficient mouse embryonic fibroblasts, overexpression of p65, a subunit of NF-kappaB, inhibited BMP2- and Smad-induced reporter activity in a dose-dependent manner. Furthermore, this p65-mediated inhibition of BMP2- and Smad-responsive promoter activity was restored after inhibition of NF-kappaB by the overexpression of the dominant negative IkappaBalpha. Although TNFalpha failed to affect receptor-dependent formation of the Smad1-Smad4 complex, p65 associated with the complex. Chromatin immunoprecipitation and electrophoresis mobility shift assays revealed that TNFalpha suppressed the DNA binding of Smad proteins to the target gene. Importantly, the specific NF-kappaB inhibitor, BAY11-7082, abolished these phenomena. These results suggest that TNFalpha inhibits BMP signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB.
Figures
Similar articles
-
Inhibition of BMP2-induced bone formation by the p65 subunit of NF-κB via an interaction with Smad4.Mol Endocrinol. 2014 Sep;28(9):1460-70. doi: 10.1210/me.2014-1094. Epub 2014 Jul 16. Mol Endocrinol. 2014. PMID: 25029242 Free PMC article.
-
Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB.J Bone Miner Res. 2007 May;22(5):646-55. doi: 10.1359/jbmr.070121. J Bone Miner Res. 2007. PMID: 17266397
-
A small nuclear acidic protein (MTI-II, Zn2+-binding protein, parathymosin) attenuates TNF-α inhibition of BMP-induced osteogenesis by enhancing accessibility of the Smad4-NF-κB p65 complex to Smad binding element.Mol Cell Biochem. 2020 Jun;469(1-2):133-142. doi: 10.1007/s11010-020-03734-6. Epub 2020 Apr 18. Mol Cell Biochem. 2020. PMID: 32304006
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
[The role of Smads and related transcription factors in the signal transduction of bone morphogenetic protein inducing bone formation].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2003 Sep;17(5):359-62. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2003. PMID: 14551929 Review. Chinese.
Cited by
-
Anti-cancer IAP antagonists promote bone metastasis: a cautionary tale.J Bone Miner Metab. 2013 Sep;31(5):496-506. doi: 10.1007/s00774-013-0479-0. Epub 2013 Jun 6. J Bone Miner Metab. 2013. PMID: 23740289 Free PMC article. Review.
-
Glucocorticoid-induced leucine zipper (GILZ) antagonizes TNF-α inhibition of mesenchymal stem cell osteogenic differentiation.PLoS One. 2012;7(3):e31717. doi: 10.1371/journal.pone.0031717. Epub 2012 Mar 2. PLoS One. 2012. PMID: 22396737 Free PMC article.
-
Involvement of PRIP, phospholipase C-related, but catalytically inactive protein, in bone formation.J Biol Chem. 2011 Sep 2;286(35):31032-31042. doi: 10.1074/jbc.M111.235903. Epub 2011 Jul 11. J Biol Chem. 2011. PMID: 21757756 Free PMC article.
-
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation.Cell Death Dis. 2014 Apr 17;5(4):e1187. doi: 10.1038/cddis.2014.101. Cell Death Dis. 2014. PMID: 24743742 Free PMC article.
-
Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling.J Biomed Sci. 2017 Feb 7;24(1):11. doi: 10.1186/s12929-017-0321-4. J Biomed Sci. 2017. PMID: 28173811 Free PMC article.
References
-
- Urist M. R. (1965) Science 150, 893–899 - PubMed
-
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Science 242, 1528–1534 - PubMed
-
- Miyazono K., Maeda S., Imamura T. (2005) Cytokine Growth Factor Rev. 16, 251–263 - PubMed
-
- Massagué J., Seoane J., Wotton D. (2005) Genes Dev. 19, 2783–2810 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

