Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis
- PMID: 19773385
- PMCID: PMC2768913
- DOI: 10.1105/tpc.109.068635
Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis
Abstract
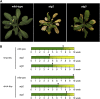
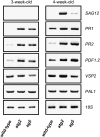
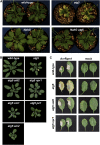
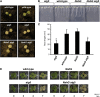
Autophagy is an evolutionarily conserved intracellular process for vacuolar degradation of cytoplasmic components. In higher plants, autophagy defects result in early senescence and excessive immunity-related programmed cell death (PCD) irrespective of nutrient conditions; however, the mechanisms by which cells die in the absence of autophagy have been unclear. Here, we demonstrate a conserved requirement for salicylic acid (SA) signaling for these phenomena in autophagy-defective mutants (atg mutants). The atg mutant phenotypes of accelerated PCD in senescence and immunity are SA signaling dependent but do not require intact jasmonic acid or ethylene signaling pathways. Application of an SA agonist induces the senescence/cell death phenotype in SA-deficient atg mutants but not in atg npr1 plants, suggesting that the cell death phenotypes in the atg mutants are dependent on the SA signal transducer NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1. We also show that autophagy is induced by the SA agonist. These findings imply that plant autophagy operates a novel negative feedback loop modulating SA signaling to negatively regulate senescence and immunity-related PCD.
Figures


Similar articles
-
Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens.Plant J. 2011 Jun;66(5):818-30. doi: 10.1111/j.1365-313X.2011.04546.x. Epub 2011 Apr 4. Plant J. 2011. PMID: 21332848
-
Ethylene and jasmonic acid signaling affect the NPR1-independent expression of defense genes without impacting resistance to Pseudomonas syringae and Peronospora parasitica in the Arabidopsis ssi1 mutant.Mol Plant Microbe Interact. 2003 Jul;16(7):588-99. doi: 10.1094/MPMI.2003.16.7.588. Mol Plant Microbe Interact. 2003. PMID: 12848424
-
The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens.Plant J. 2002 May;30(4):467-80. doi: 10.1046/j.1365-313x.2002.01300.x. Plant J. 2002. PMID: 12028576
-
NPR1: the spider in the web of induced resistance signaling pathways.Curr Opin Plant Biol. 2004 Aug;7(4):456-64. doi: 10.1016/j.pbi.2004.05.006. Curr Opin Plant Biol. 2004. PMID: 15231270 Review.
-
Plant autophagy puts the brakes on cell death by controlling salicylic acid signaling.Autophagy. 2010 Jan;6(1):192-3. doi: 10.4161/auto.6.1.10843. Epub 2010 Jan 4. Autophagy. 2010. PMID: 20023431 Review.
Cited by
-
Senescence-Associated Vacuoles, a Specific Lytic Compartment for Degradation of Chloroplast Proteins?Plants (Basel). 2014 Nov 11;3(4):498-512. doi: 10.3390/plants3040498. Plants (Basel). 2014. PMID: 27135516 Free PMC article. Review.
-
Autophagy contributes to nighttime energy availability for growth in Arabidopsis.Plant Physiol. 2013 Apr;161(4):1682-93. doi: 10.1104/pp.113.215632. Epub 2013 Mar 1. Plant Physiol. 2013. PMID: 23457226 Free PMC article.
-
Phosphorylation of ATG18a by BAK1 suppresses autophagy and attenuates plant resistance against necrotrophic pathogens.Autophagy. 2021 Sep;17(9):2093-2110. doi: 10.1080/15548627.2020.1810426. Epub 2020 Aug 26. Autophagy. 2021. PMID: 32804012 Free PMC article.
-
Autophagy-virus interplay in plants: from antiviral recognition to proviral manipulation.Mol Plant Pathol. 2019 Sep;20(9):1211-1216. doi: 10.1111/mpp.12852. Epub 2019 Aug 9. Mol Plant Pathol. 2019. PMID: 31397085 Free PMC article. Review.
-
Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence.Plant Cell. 2010 May;22(5):1463-82. doi: 10.1105/tpc.110.075333. Epub 2010 May 4. Plant Cell. 2010. PMID: 20442372 Free PMC article.
References
-
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152. - PubMed
-
- Barth, H., Meiling-Wesse, K., Epple, U.D., and Thumm, M. (2001). Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 508 23–28. - PubMed
-
- Bassham, D.C., Laporte, M., Marty, F., Moriyasu, Y., Ohsumi, Y., Olsen, L.J., and Yoshimoto, K. (2006). Autophagy in development and stress responses of plants. Autophagy 2 2–11. - PubMed
-
- Cao, H., Glazebrook, J., Clarke, J.D., Volko, S., and Dong, X. (1997). The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 57–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

