Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload
- PMID: 19707545
- PMCID: PMC2727794
- DOI: 10.1371/journal.pone.0006743
Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload
Erratum in
- PLoS One. 2009;4(9). doi: 10.1371/annotation/818d7cc6-3ec0-4fc5-82e1-8e9b6ceca336. Morano, Rainer Dietz Ingo [corrected to Dietz, Rainer]; Morano, Ingo [added]
Abstract
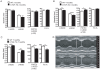
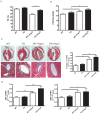
Connective tissue growth factor (CTGF) is a secreted protein that is strongly induced in human and experimental heart failure. CTGF is said to be profibrotic; however, the precise function of CTGF is unclear. We generated transgenic mice and rats with cardiomyocyte-specific CTGF overexpression (CTGF-TG). To investigate CTGF as a fibrosis inducer, we performed morphological and gene expression analyses of CTGF-TG mice and rat hearts under basal conditions and after stimulation with angiotensin II (Ang II) or isoproterenol, respectively. Surprisingly, cardiac tissues of both models did not show increased fibrosis or enhanced gene expression of fibrotic markers. In contrast to controls, Ang II treated CTGF-TG mice displayed preserved cardiac function. However, CTGF-TG mice developed age-dependent cardiac dysfunction at the age of 7 months. CTGF related heart failure was associated with Akt and JNK activation, but not with the induction of natriuretic peptides. Furthermore, cardiomyocytes from CTGF-TG mice showed unaffected cellular contractility and an increased Ca(2+) reuptake from sarcoplasmatic reticulum. In an ischemia/reperfusion model CTGF-TG hearts did not differ from controls.Our data suggest that CTGF itself does not induce cardiac fibrosis. Moreover, it is involved in hypertrophy induction and cellular remodeling depending on the cardiac stress stimulus. Our new transgenic animals are valuable models for reconsideration of CTGF's profibrotic function in the heart.
Conflict of interest statement
Figures



Similar articles
-
CCN2/CTGF attenuates myocardial hypertrophy and cardiac dysfunction upon chronic pressure-overload.Int J Cardiol. 2013 Oct 3;168(3):2049-56. doi: 10.1016/j.ijcard.2013.01.165. Epub 2013 Feb 26. Int J Cardiol. 2013. PMID: 23452880
-
Overexpressed connective tissue growth factor in cardiomyocytes attenuates left ventricular remodeling induced by angiotensin II perfusion.Clin Exp Hypertens. 2017;39(2):168-174. doi: 10.1080/10641963.2016.1226893. Epub 2017 Mar 1. Clin Exp Hypertens. 2017. PMID: 28287886
-
Connective tissue growth factor/CCN2 attenuates β-adrenergic receptor responsiveness and cardiotoxicity by induction of G protein-coupled receptor kinase-5 in cardiomyocytes.Mol Pharmacol. 2013 Sep;84(3):372-83. doi: 10.1124/mol.113.087312. Epub 2013 Jun 18. Mol Pharmacol. 2013. PMID: 23778361
-
Mechanisms of novel cardioprotective functions of CCN2/CTGF in myocardial ischemia-reperfusion injury.Am J Physiol Heart Circ Physiol. 2011 Apr;300(4):H1291-302. doi: 10.1152/ajpheart.00604.2010. Epub 2010 Dec 24. Am J Physiol Heart Circ Physiol. 2011. PMID: 21186275
-
Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure.Hypertension. 2014 Jun;63(6):1235-40. doi: 10.1161/HYPERTENSIONAHA.114.03279. Epub 2014 Mar 31. Hypertension. 2014. PMID: 24688123
Cited by
-
Angiotensin II-mediated up-regulation of connective tissue growth factor promotes atrial tissue fibrosis in the canine atrial fibrillation model.Europace. 2012 Aug;14(8):1206-14. doi: 10.1093/europace/eus052. Epub 2012 Mar 27. Europace. 2012. PMID: 22454409 Free PMC article.
-
CCN2 Increases TGF-β Receptor Type II Expression in Vascular Smooth Muscle Cells: Essential Role of CCN2 in the TGF-β Pathway Regulation.Int J Mol Sci. 2021 Dec 29;23(1):375. doi: 10.3390/ijms23010375. Int J Mol Sci. 2021. PMID: 35008801 Free PMC article.
-
Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease.Nat Rev Nephrol. 2014 Dec;10(12):700-11. doi: 10.1038/nrneph.2014.184. Epub 2014 Oct 14. Nat Rev Nephrol. 2014. PMID: 25311535 Review.
-
Augmented cardiac hypertrophy in response to pressure overload in mice lacking ELTD1.PLoS One. 2012;7(5):e35779. doi: 10.1371/journal.pone.0035779. Epub 2012 May 11. PLoS One. 2012. PMID: 22606234 Free PMC article.
-
Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice.Cardiovasc Res. 2012 Dec 1;96(3):444-55. doi: 10.1093/cvr/cvs275. Epub 2012 Aug 22. Cardiovasc Res. 2012. PMID: 22918978 Free PMC article.
References
-
- Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. - PubMed
-
- Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. - PubMed
-
- Chien KR. Genomic circuits and the integrative biology of cardiac diseases. Nature. 2000;407:227–232. - PubMed
-
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. - PubMed
-
- Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, et al. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

