Laminin enhances beta(2)-adrenergic receptor stimulation of L-type Ca(2+) current via cytosolic phospholipase A(2) signalling in cat atrial myocytes
- PMID: 19703961
- PMCID: PMC2770147
- DOI: 10.1113/jphysiol.2009.179226
Laminin enhances beta(2)-adrenergic receptor stimulation of L-type Ca(2+) current via cytosolic phospholipase A(2) signalling in cat atrial myocytes
Abstract
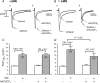
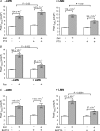
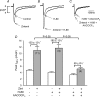
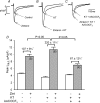
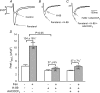
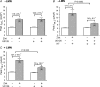
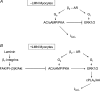
We previously reported that attachment of atrial myocytes to the extracellular matrix protein laminin (LMN), decreases adenylate cyclase (AC)/cAMP and increases beta(2)-adrenergic receptor (AR) stimulation of L-type Ca(2+) current (I(Ca,L)). This study therefore sought to determine whether LMN enhances beta(2)-AR signalling via a cAMP-independent mechanism, i.e. cytosolic phospholipase A(2) (cPLA(2)) signalling. Studies were performed on acutely isolated atrial myocytes plated on uncoated coverslips (LMN) or coverslips coated with LMN (+LMN). As previously reported, 0.1 microm zinterol (zint-beta(2)-AR) stimulation of I(Ca,L) was larger in +LMN than LMN myocytes. In +LMN myocytes, zint-beta(2)-AR stimulation of I(Ca,L) was inhibited by inhibition of cPLA(2) by arachidonyltrifluoromethyl ketone (AACOCF(3); 10 microm), inhibition of G(i) by pertussis toxin and chelation of intracellular Ca(2+) by 10 microm BAPTA-AM. In contrast to zinterol, stimulation of I(Ca,L) by fenoterol (fen-beta(2)-AR), a beta(2)-AR agonist that acts exclusively via G(s) signalling, was smaller in +LMN than LMN myocytes. Arachidonic acid (AA; 5 microm) stimulated I(Ca,L) to a similar extent in LMN and +LMN myocytes. Inhibition of cAMP-dependent protein kinase A (cAMP/PKA) by either 5 mum H89 or 1 microm KT5720 in LMN myocytes mimicked the effects of +LMN myocytes to enhance zint-beta(2)-AR stimulation of I(Ca,L), which was blocked by 10 microm AACOCF(3). In contrast, H89 inhibited fen-beta(2)-AR stimulation of I(Ca,L), which was unchanged by AACOCF(3). Inhibition of ERK1/2 by 1 microm U0126 inhibited zint-beta(2)-AR stimulation of I(Ca,L) in +LMN myocytes and LMN myocytes in which cAMP/PKA was inhibited by KT5720. In LMN myocytes, cytochalasin D prevented inhibition of cAMP/PKA from enhancing zint-beta(2)-AR stimulation of I(Ca,L). We conclude that LMN enhances zint-beta(2)-AR stimulation of I(Ca,L) via G(i)/ERK1/2/cPLA(2)/AA signalling which is activated by concomitant inhibition of cAMP/PKA signalling and dependent on the actin cytoskeleton. These findings provide new insight into the cellular mechanisms by which the extracellular matrix can remodel beta(2)-AR signalling in atrial muscle.
Figures
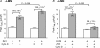
Similar articles
-
Inhibition of cAMP-Dependent PKA Activates β2-Adrenergic Receptor Stimulation of Cytosolic Phospholipase A2 via Raf-1/MEK/ERK and IP3-Dependent Ca2+ Signaling in Atrial Myocytes.PLoS One. 2016 Dec 15;11(12):e0168505. doi: 10.1371/journal.pone.0168505. eCollection 2016. PLoS One. 2016. PMID: 27977772 Free PMC article.
-
Laminin acts via focal adhesion kinase/phosphatidylinositol-3' kinase/protein kinase B to down-regulate beta1-adrenergic receptor signalling in cat atrial myocytes.J Physiol. 2009 Feb 1;587(3):541-50. doi: 10.1113/jphysiol.2008.163824. Epub 2008 Dec 8. J Physiol. 2009. PMID: 19064616 Free PMC article.
-
Nitric oxide signalling by selective beta(2)-adrenoceptor stimulation prevents ACh-induced inhibition of beta(2)-stimulated Ca(2+) current in cat atrial myocytes.J Physiol. 2002 Aug 1;542(Pt 3):711-23. doi: 10.1113/jphysiol.2002.023341. J Physiol. 2002. PMID: 12154173 Free PMC article.
-
Recent advances in cardiac beta(2)-adrenergic signal transduction.Circ Res. 1999 Nov 26;85(11):1092-100. doi: 10.1161/01.res.85.11.1092. Circ Res. 1999. PMID: 10571541 Review.
-
Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels.Comp Biochem Physiol A Mol Integr Physiol. 2005 Oct;142(2):136-43. doi: 10.1016/j.cbpb.2005.04.012. Epub 2005 May 31. Comp Biochem Physiol A Mol Integr Physiol. 2005. PMID: 15927494 Review.
Cited by
-
Integrin and GPCR Crosstalk in the Regulation of ASM Contraction Signaling in Asthma.J Allergy (Cairo). 2012;2012:341282. doi: 10.1155/2012/341282. Epub 2012 Sep 29. J Allergy (Cairo). 2012. PMID: 23056062 Free PMC article.
-
Inhibition of cAMP-Dependent PKA Activates β2-Adrenergic Receptor Stimulation of Cytosolic Phospholipase A2 via Raf-1/MEK/ERK and IP3-Dependent Ca2+ Signaling in Atrial Myocytes.PLoS One. 2016 Dec 15;11(12):e0168505. doi: 10.1371/journal.pone.0168505. eCollection 2016. PLoS One. 2016. PMID: 27977772 Free PMC article.
-
Regulation of cardiac alternans by β-adrenergic signaling pathways.Am J Physiol Heart Circ Physiol. 2012 Oct 15;303(8):H1047-56. doi: 10.1152/ajpheart.00384.2012. Epub 2012 Aug 17. Am J Physiol Heart Circ Physiol. 2012. PMID: 22904161 Free PMC article.
References
-
- Ait-Mamar B, Cailleret M, Rucker-Martin C, Bouabdallah A, Candiani G, Adamy D, Duvaldestin P, Pecker F, Defer N, Pavoine C. The cytosolic phospholipase A2 pathway, a safeguard of β2-adrenergic cardiac effects in rat. J Biol Chem. 2005;280:18881–18890. - PubMed
-
- Bonventre JV. The 85-kD cytosolic phospholipase A2 knockout mouse; a new tool for physiology and cell biology. J Am Soc Nephrol. 1999;10:404–412. - PubMed
-
- Bristow MR, Ginsberg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson EB. β1- and β2-adrenergic-receptor subpopulation in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. - PubMed
-
- Carter SB. Effects of cytochalasins on mammalian cells. Nature. 1967;213:261–264. - PubMed
-
- Chesley A, Lundberg MS, Asai T, Xiao R-P, Ohtani S, Lakatta EG, Crow MT. The β2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

