Basal cells as stem cells of the mouse trachea and human airway epithelium
- PMID: 19625615
- PMCID: PMC2714281
- DOI: 10.1073/pnas.0906850106
Basal cells as stem cells of the mouse trachea and human airway epithelium
Abstract
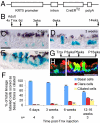
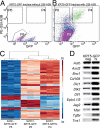
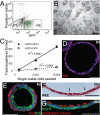
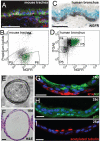
The pseudostratified epithelium of the mouse trachea and human airways contains a population of basal cells expressing Trp-63 (p63) and cytokeratins 5 (Krt5) and Krt14. Using a KRT5-CreER(T2) transgenic mouse line for lineage tracing, we show that basal cells generate differentiated cells during postnatal growth and in the adult during both steady state and epithelial repair. We have fractionated mouse basal cells by FACS and identified 627 genes preferentially expressed in a basal subpopulation vs. non-BCs. Analysis reveals potential mechanisms regulating basal cells and allows comparison with other epithelial stem cells. To study basal cell behaviors, we describe a simple in vitro clonal sphere-forming assay in which mouse basal cells self-renew and generate luminal cells, including differentiated ciliated cells, in the absence of stroma. The transcriptional profile identified 2 cell-surface markers, ITGA6 and NGFR, which can be used in combination to purify human lung basal cells by FACS. Like those from the mouse trachea, human airway basal cells both self-renew and generate luminal daughters in the sphere-forming assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cell surface marker profiling of human tracheal basal cells reveals distinct subpopulations, identifies MST1/MSP as a mitogenic signal, and identifies new biomarkers for lung squamous cell carcinomas.Respir Res. 2014 Dec 31;15(1):160. doi: 10.1186/s12931-014-0160-8. Respir Res. 2014. PMID: 25551685 Free PMC article.
-
Spatial-Temporal Lineage Restrictions of Embryonic p63+ Progenitors Establish Distinct Stem Cell Pools in Adult Airways.Dev Cell. 2018 Mar 26;44(6):752-761.e4. doi: 10.1016/j.devcel.2018.03.001. Dev Cell. 2018. PMID: 29587145 Free PMC article.
-
Dorso-ventral heterogeneity in tracheal basal stem cells.Biol Open. 2021 Sep 15;10(9):bio058676. doi: 10.1242/bio.058676. Epub 2021 Sep 14. Biol Open. 2021. PMID: 34396394 Free PMC article.
-
Characterization, isolation, expansion and clinical therapy of human corneal epithelial stem/progenitor cells.J Stem Cells. 2014;9(2):79-91. J Stem Cells. 2014. PMID: 25158157 Review.
-
Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair.Cold Spring Harb Symp Quant Biol. 2008;73:291-5. doi: 10.1101/sqb.2008.73.037. Epub 2008 Nov 21. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19028985 Review.
Cited by
-
Regenerative potential of human airway stem cells in lung epithelial engineering.Biomaterials. 2016 Nov;108:111-9. doi: 10.1016/j.biomaterials.2016.08.055. Epub 2016 Sep 4. Biomaterials. 2016. PMID: 27622532 Free PMC article.
-
An organoids biobank for recapitulating tumor heterogeneity and personalized medicine.Chin J Cancer Res. 2020 Jun;32(3):408-413. doi: 10.21147/j.issn.1000-9604.2020.03.12. Chin J Cancer Res. 2020. PMID: 32694905 Free PMC article. No abstract available.
-
Cilia-related gene signature in the nasal mucosa correlates with disease severity and outcomes in critical respiratory syncytial virus bronchiolitis.Front Immunol. 2022 Sep 23;13:924792. doi: 10.3389/fimmu.2022.924792. eCollection 2022. Front Immunol. 2022. PMID: 36211387 Free PMC article.
-
Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice.PLoS One. 2012;7(3):e33529. doi: 10.1371/journal.pone.0033529. Epub 2012 Mar 28. PLoS One. 2012. PMID: 22470452 Free PMC article.
-
A microfluidic device to apply shear stresses to polarizing ciliated airway epithelium using air flow.Biomicrofluidics. 2014 Nov 14;8(6):064104. doi: 10.1063/1.4901930. eCollection 2014 Nov. Biomicrofluidics. 2014. PMID: 25553181 Free PMC article.
References
-
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. - PubMed
-
- Daniely Y, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–C181. - PubMed
-
- Schoch KG, et al. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L631–L642. - PubMed
-
- Nakajima M, et al. Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathol Int. 1998;48:944–953. - PubMed
-
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157(6 Pt 1):2000–2006. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

