Toll-like receptor signaling controls reactivation of KSHV from latency
- PMID: 19564611
- PMCID: PMC2710638
- DOI: 10.1073/pnas.0905316106
Toll-like receptor signaling controls reactivation of KSHV from latency
Abstract
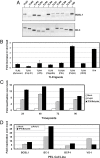
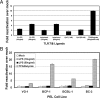
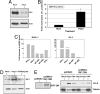
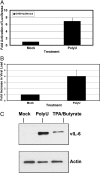
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of Kaposi's sarcoma, primary effusion lymphoma (PEL), and multicentric Castleman's disease. Like other herpesviruses, KSHV establishes life-long latency in the human host with intermittent periods of reactivation. Physiological triggers of herpesviral reactivation are poorly defined. Toll-like receptors (TLRs) recognize pathogens and are vital for the host innate immune response. We screened multiple TLR agonists for their ability to initiate KSHV replication in latently infected PEL. Agonists specific for TLR7/8 reactivated latent KSHV and induced viral lytic gene transcription and replication. Furthermore, vesicular stomatitis virus (VSV), a bonafide physiological activator of TLR7/8, also reactivated KSHV from latency. This demonstrates that secondary pathogen infection of latently infected cells can reactivate KSHV. Human herpesviruses establish life-long latency in the host, and it is plausible that a latently infected cell will encounter multiple pathogens during its lifetime and that these encounters lead to episodic reactivation. Our findings have broad implications for physiological triggers of latent viral infections, such as herpesviral reactivation and persistence in the host.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response.J Virol. 2014 Aug;88(16):9245-59. doi: 10.1128/JVI.00841-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899179 Free PMC article.
-
Expression and Subcellular Localization of the Kaposi's Sarcoma-Associated Herpesvirus K15P Protein during Latency and Lytic Reactivation in Primary Effusion Lymphoma Cells.J Virol. 2017 Oct 13;91(21):e01370-17. doi: 10.1128/JVI.01370-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835496 Free PMC article.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
-
Chromatin remodeling controls Kaposi's sarcoma-associated herpesvirus reactivation from latency.PLoS Pathog. 2018 Sep 13;14(9):e1007267. doi: 10.1371/journal.ppat.1007267. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30212584 Free PMC article.
-
KSHV: pathways to tumorigenesis and persistent infection.Adv Virus Res. 2014;88:111-59. doi: 10.1016/B978-0-12-800098-4.00002-7. Adv Virus Res. 2014. PMID: 24373311 Free PMC article. Review.
Cited by
-
TLR-TRIF pathway enhances the expression of KSHV replication and transcription activator.J Biol Chem. 2013 Jul 12;288(28):20435-42. doi: 10.1074/jbc.M113.487421. Epub 2013 May 30. J Biol Chem. 2013. PMID: 23723066 Free PMC article.
-
Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy.J Clin Invest. 2016 Sep 1;126(9):3165-75. doi: 10.1172/JCI84418. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584730 Free PMC article. Review.
-
NCOA2 promotes lytic reactivation of Kaposi's sarcoma-associated herpesvirus by enhancing the expression of the master switch protein RTA.PLoS Pathog. 2019 Nov 21;15(11):e1008160. doi: 10.1371/journal.ppat.1008160. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31751430 Free PMC article.
-
Reactive oxygen species hydrogen peroxide mediates Kaposi's sarcoma-associated herpesvirus reactivation from latency.PLoS Pathog. 2011 May;7(5):e1002054. doi: 10.1371/journal.ppat.1002054. Epub 2011 May 19. PLoS Pathog. 2011. PMID: 21625536 Free PMC article.
-
The gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response.J Virol. 2014 Aug;88(16):9245-59. doi: 10.1128/JVI.00841-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899179 Free PMC article.
References
-
- Chang Y, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Whitby D, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. - PubMed
-
- Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. - PubMed
-
- Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

