Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein
- PMID: 19564338
- PMCID: PMC2755666
- DOI: 10.1074/jbc.M109.032599
Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein
Abstract
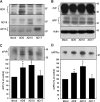
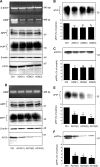
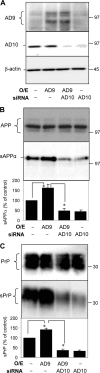
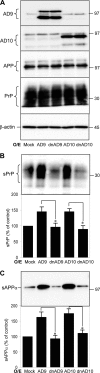
The cellular prion protein (PrP(C)) is essential for the pathogenesis and transmission of prion diseases. PrP(C) is bound to the plasma membrane via a glycosylphosphatidylinositol anchor, although a secreted, soluble form has also been identified. Previously we reported that PrP(C) is subject to ectodomain shedding from the membrane by zinc metalloproteinases with a similar inhibition profile to those involved in shedding the amyloid precursor protein. Here we have used gain-of-function (overexpression) and loss-of-function (small interfering RNA knockdown) experiments in cells to identify the ADAMs (a disintegrin and metalloproteinases) involved in the ectodomain shedding of PrP(C). These experiments revealed that ADAM9 and ADAM10, but not ADAM17, are involved in the shedding of PrP(C) and that ADAM9 exerts its effect on PrP(C) shedding via ADAM10. Using dominant negative, catalytically inactive mutants, we show that the catalytic activity of ADAM9 is required for its effect on ADAM10. Mass spectrometric analysis revealed that ADAM10, but not ADAM9, cleaved PrP between Gly(228) and Arg(229), three residues away from the site of glycosylphosphatidylinositol anchor attachment. The shedding of another membrane protein, the amyloid precursor protein beta-secretase BACE1, by ADAM9 is also mediated via ADAM10. Furthermore, we show that pharmacological inhibition of PrP(C) shedding or activation of both PrP(C) and PrP(Sc) shedding by ADAM10 overexpression in scrapie-infected neuroblastoma N2a cells does not alter the formation of proteinase K-resistant PrP(Sc). Collectively, these data indicate that although PrP(C) can be shed through the action of ADAM family members, modulation of PrP(C) or PrP(Sc) ectodomain shedding does not regulate prion conversion.
Figures



Similar articles
-
The disintegrin ADAM9 indirectly contributes to the physiological processing of cellular prion by modulating ADAM10 activity.J Biol Chem. 2005 Dec 9;280(49):40624-31. doi: 10.1074/jbc.M506069200. Epub 2005 Oct 18. J Biol Chem. 2005. PMID: 16236709
-
Roles of endoproteolytic α-cleavage and shedding of the prion protein in neurodegeneration.FEBS J. 2013 Sep;280(18):4338-47. doi: 10.1111/febs.12196. Epub 2013 Mar 13. FEBS J. 2013. PMID: 23413979 Review.
-
A new paradigm for enzymatic control of α-cleavage and β-cleavage of the prion protein.J Biol Chem. 2014 Jan 10;289(2):803-13. doi: 10.1074/jbc.M113.502351. Epub 2013 Nov 18. J Biol Chem. 2014. PMID: 24247244 Free PMC article.
-
A disintegrin and metalloproteinase (ADAM)-mediated ectodomain shedding of ADAM10.J Neurochem. 2009 Mar;108(6):1464-79. doi: 10.1111/j.1471-4159.2009.05907.x. Epub 2009 Jan 22. J Neurochem. 2009. PMID: 19183255
-
The "A Disintegrin And Metalloproteases" ADAM10 and ADAM17: novel drug targets with therapeutic potential?Eur J Cell Biol. 2011 Jun-Jul;90(6-7):527-35. doi: 10.1016/j.ejcb.2010.11.005. Epub 2010 Dec 30. Eur J Cell Biol. 2011. PMID: 21194787 Review.
Cited by
-
Proteolytic processing of the prion protein in health and disease.Am J Neurodegener Dis. 2012;1(1):15-31. Epub 2012 May 15. Am J Neurodegener Dis. 2012. PMID: 23383379 Free PMC article.
-
Prion Protein: The Molecule of Many Forms and Faces.Int J Mol Sci. 2022 Jan 22;23(3):1232. doi: 10.3390/ijms23031232. Int J Mol Sci. 2022. PMID: 35163156 Free PMC article. Review.
-
Neuroprotective effect and potential of cellular prion protein and its cleavage products for treatment of neurodegenerative disorders part II: strategies for therapeutics development.Expert Rev Neurother. 2021 Sep;21(9):983-991. doi: 10.1080/14737175.2021.1965882. Epub 2021 Sep 2. Expert Rev Neurother. 2021. PMID: 34470554 Free PMC article.
-
The extracellular regulated kinase-1 (ERK1) controls regulated alpha-secretase-mediated processing, promoter transactivation, and mRNA levels of the cellular prion protein.J Biol Chem. 2011 Aug 19;286(33):29192-29206. doi: 10.1074/jbc.M110.208249. Epub 2011 May 17. J Biol Chem. 2011. PMID: 21586567 Free PMC article.
-
New implications for prion diseases therapy and prophylaxis.Front Mol Neurosci. 2024 Mar 4;17:1324702. doi: 10.3389/fnmol.2024.1324702. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38500676 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

