Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases
- PMID: 19473963
- PMCID: PMC2782016
- DOI: 10.1074/jbc.M109.016816
Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases
Abstract
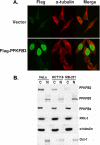
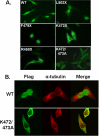
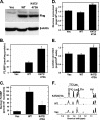
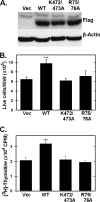
The regulation of metabolism and growth must be tightly coupled to guarantee the efficient use of energy and anabolic substrates throughout the cell cycle. Fructose 2,6-bisphosphate (Fru-2,6-BP) is an allosteric activator of 6-phosphofructo-1-kinase (PFK-1), a rate-limiting enzyme and essential control point in glycolysis. The concentration of Fru-2,6-BP in mammalian cells is set by four 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFB1-4), which interconvert fructose 6-phosphate and Fru-2,6-BP. The relative functions of the PFKFB3 and PFKFB4 enzymes are of particular interest because they are activated in human cancers and increased by mitogens and low oxygen. We examined the cellular localization of PFKFB3 and PFKFB4 and unexpectedly found that whereas PFKFB4 localized to the cytoplasm (i.e. the site of glycolysis), PFKFB3 localized to the nucleus. We then overexpressed PFKFB3 and observed no change in glucose metabolism but rather a marked increase in cell proliferation. These effects on proliferation were completely abrogated by mutating either the active site or nuclear localization residues of PFKFB3, demonstrating a requirement for nuclear delivery of Fru-2,6-BP. Using protein array analyses, we then found that ectopic expression of PFKFB3 increased the expression of several key cell cycle proteins, including cyclin-dependent kinase (Cdk)-1, Cdc25C, and cyclin D3 and decreased the expression of the cell cycle inhibitor p27, a universal inhibitor of Cdk-1 and the cell cycle. We also observed that the addition of Fru-2,6-BP to HeLa cell lysates increased the phosphorylation of the Cdk-specific Thr-187 site of p27. Taken together, these observations demonstrate an unexpected role for PFKFB3 in nuclear signaling and indicate that Fru-2,6-BP may couple the activation of glucose metabolism with cell proliferation.
Figures

Similar articles
-
6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle progression and suppresses apoptosis via Cdk1-mediated phosphorylation of p27.Cell Death Dis. 2014 Jul 17;5(7):e1337. doi: 10.1038/cddis.2014.292. Cell Death Dis. 2014. PMID: 25032860 Free PMC article.
-
Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes.Cell Signal. 2017 Jun;34:23-37. doi: 10.1016/j.cellsig.2017.02.019. Epub 2017 Feb 22. Cell Signal. 2017. PMID: 28235572
-
TGF-β1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells.FEBS J. 2017 Oct;284(20):3437-3454. doi: 10.1111/febs.14201. Epub 2017 Sep 10. FEBS J. 2017. PMID: 28834297
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 and 4: A pair of valves for fine-tuning of glucose metabolism in human cancer.Mol Metab. 2019 Feb;20:1-13. doi: 10.1016/j.molmet.2018.11.013. Epub 2018 Dec 5. Mol Metab. 2019. PMID: 30553771 Free PMC article. Review.
-
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis.Curr Opin Clin Nutr Metab Care. 2006 Sep;9(5):535-9. doi: 10.1097/01.mco.0000241661.15514.fb. Curr Opin Clin Nutr Metab Care. 2006. PMID: 16912547 Review.
Cited by
-
The Warburg Effect in Endothelial Cells and its Potential as an Anti-angiogenic Target in Cancer.Front Cell Dev Biol. 2018 Sep 11;6:100. doi: 10.3389/fcell.2018.00100. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30255018 Free PMC article. Review.
-
Canonical and Non-Canonical Roles of PFKFB3 in Brain Tumors.Cells. 2021 Oct 27;10(11):2913. doi: 10.3390/cells10112913. Cells. 2021. PMID: 34831136 Free PMC article. Review.
-
Endocervical trophoblast for interrogating the fetal genome and assessing pregnancy health at five weeks.Eur J Med Genet. 2019 Aug;62(8):103690. doi: 10.1016/j.ejmg.2019.103690. Epub 2019 Jun 18. Eur J Med Genet. 2019. PMID: 31226440 Free PMC article.
-
Glucose and glutamine metabolism control by APC and SCF during the G1-to-S phase transition of the cell cycle.J Physiol Biochem. 2014 Jun;70(2):569-81. doi: 10.1007/s13105-014-0328-1. Epub 2014 Mar 7. J Physiol Biochem. 2014. PMID: 24604252 Review.
-
Long noncoding RNA AGPG regulates PFKFB3-mediated tumor glycolytic reprogramming.Nat Commun. 2020 Mar 20;11(1):1507. doi: 10.1038/s41467-020-15112-3. Nat Commun. 2020. PMID: 32198345 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

