Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice
- PMID: 19420076
- PMCID: PMC2704767
- DOI: 10.1128/JVI.02207-08
Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice
Abstract
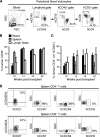
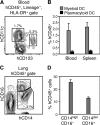
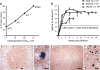
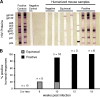
The generation of humanized BLT mice by the cotransplantation of human fetal thymus and liver tissues and CD34(+) fetal liver cells into nonobese diabetic/severe combined immunodeficiency mice allows for the long-term reconstitution of a functional human immune system, with human T cells, B cells, dendritic cells, and monocytes/macrophages repopulating mouse tissues. Here, we show that humanized BLT mice sustained high-level disseminated human immunodeficiency virus (HIV) infection, resulting in CD4(+) T-cell depletion and generalized immune activation. Following infection, HIV-specific humoral responses were present in all mice by 3 months, and HIV-specific CD4(+) and CD8(+) T-cell responses were detected in the majority of mice tested after 9 weeks of infection. Despite robust HIV-specific responses, however, viral loads remained elevated in infected BLT mice, raising the possibility that these responses are dysfunctional. The increased T-cell expression of the negative costimulator PD-1 recently has been postulated to contribute to T-cell dysfunction in chronic HIV infection. As seen in human infection, both CD4(+) and CD8(+) T cells demonstrated increased PD-1 expression in HIV-infected BLT mice, and PD-1 levels in these cells correlated positively with viral load and inversely with CD4(+) cell levels. The ability of humanized BLT mice to generate both cellular and humoral immune responses to HIV will allow the further investigation of human HIV-specific immune responses in vivo and suggests that these mice are able to provide a platform to assess candidate HIV vaccines and other immunotherapeutic strategies.
Figures





Similar articles
-
Innate Immune Reconstitution in Humanized Bone Marrow-Liver-Thymus (HuBLT) Mice Governs Adaptive Cellular Immune Function and Responses to HIV-1 Infection.Front Immunol. 2021 May 26;12:667393. doi: 10.3389/fimmu.2021.667393. eCollection 2021. Front Immunol. 2021. PMID: 34122425 Free PMC article.
-
Immunization of BLT Humanized Mice Redirects T Cell Responses to Gag and Reduces Acute HIV-1 Viremia.J Virol. 2019 Sep 30;93(20):e00814-19. doi: 10.1128/JVI.00814-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375576 Free PMC article.
-
PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads.PLoS One. 2013 Oct 21;8(10):e77780. doi: 10.1371/journal.pone.0077780. eCollection 2013. PLoS One. 2013. PMID: 24204962 Free PMC article.
-
HIV-specific CD8⁺ T-cell immunity in humanized bone marrow-liver-thymus mice.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S150-4. doi: 10.1093/infdis/jit320. J Infect Dis. 2013. PMID: 24151322 Free PMC article. Review.
-
Cell-to-Cell Spread of HIV and Viral Pathogenesis.Adv Virus Res. 2016;95:43-85. doi: 10.1016/bs.aivir.2016.03.001. Epub 2016 Apr 4. Adv Virus Res. 2016. PMID: 27112280 Review.
Cited by
-
Graft versus host disease in the bone marrow, liver and thymus humanized mouse model.PLoS One. 2012;7(9):e44664. doi: 10.1371/journal.pone.0044664. Epub 2012 Sep 5. PLoS One. 2012. PMID: 22957096 Free PMC article.
-
Innate Immune Reconstitution in Humanized Bone Marrow-Liver-Thymus (HuBLT) Mice Governs Adaptive Cellular Immune Function and Responses to HIV-1 Infection.Front Immunol. 2021 May 26;12:667393. doi: 10.3389/fimmu.2021.667393. eCollection 2021. Front Immunol. 2021. PMID: 34122425 Free PMC article.
-
Use of pediatric thymus to humanize mice for HIV-1 mucosal transmission.Sci Rep. 2023 Oct 10;13(1):17067. doi: 10.1038/s41598-023-44366-2. Sci Rep. 2023. PMID: 37816950 Free PMC article.
-
Human Cytomegalovirus Induces Cellular and Humoral Virus-specific Immune Responses in Humanized BLT Mice.Sci Rep. 2017 Apr 20;7(1):937. doi: 10.1038/s41598-017-01051-5. Sci Rep. 2017. PMID: 28428537 Free PMC article.
-
Longitudinal imaging of HIV-1 spread in humanized mice with parallel 3D immunofluorescence and electron tomography.Elife. 2017 Feb 15;6:e23282. doi: 10.7554/eLife.23282. Elife. 2017. PMID: 28198699 Free PMC article.
References
-
- Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193169-180. - PMC - PubMed
-
- Baenziger, S., R. Tussiwand, E. Schlaepfer, L. Mazzucchelli, M. Heikenwalder, M. O. Kurrer, S. Behnke, J. Frey, A. Oxenius, H. Joller, A. Aguzzi, M. G. Manz, and R. F. Speck. 2006. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/− γc−/− mice. Proc. Natl. Acad. Sci. USA 10315951-15956. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AI058857/AI/NIAID NIH HHS/United States
- R21 AI069458/AI/NIAID NIH HHS/United States
- T32 RR007000/RR/NCRR NIH HHS/United States
- R01 A1067077/PHS HHS/United States
- T32 RR07000/RR/NCRR NIH HHS/United States
- P01 AI078897/AI/NIAID NIH HHS/United States
- AI069458/AI/NIAID NIH HHS/United States
- R01 AI064930/AI/NIAID NIH HHS/United States
- AI064930/AI/NIAID NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- P51 RR00168/RR/NCRR NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- K08AI058857/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

