The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA
- PMID: 19362020
- PMCID: PMC2803103
- DOI: 10.1016/j.immuni.2009.02.005
The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA
Abstract
The nucleotide-binding domain and leucine-rich-repeat-containing (NLR) family of pattern-recognition molecules mediate host immunity to various pathogenic stimuli. However, in vivo evidence for the involvement of NLR proteins in viral sensing has not been widely investigated and remains controversial. As a test of the physiologic role of the NLR molecule NLRP3 during RNA viral infection, we explored the in vivo role of NLRP3 inflammasome components during influenza virus infection. Mice lacking Nlrp3, Pycard, or caspase-1, but not Nlrc4, exhibited dramatically increased mortality and a reduced immune response after exposure to the influenza virus. Utilizing analogs of dsRNA (poly(I:C)) and ssRNA (ssRNA40), we demonstrated that an NLRP3-mediated response could be activated by RNA species. Mechanistically, NLRP3 inflammasome activation by the influenza virus was dependent on lysosomal maturation and reactive oxygen species (ROS). Inhibition of ROS induction eliminated IL-1beta production in animals during influenza infection. Together, these data place the NLRP3 inflammasome as an essential component in host defense against influenza infection through the sensing of viral RNA.
Figures
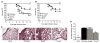
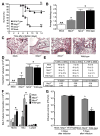
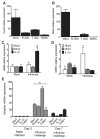
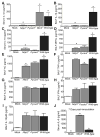
Comment in
-
Fighting the flu with inflammasome signaling.Immunity. 2009 Apr 17;30(4):476-8. doi: 10.1016/j.immuni.2009.03.011. Immunity. 2009. PMID: 19371712
Similar articles
-
NS1 Protein of 2009 Pandemic Influenza A Virus Inhibits Porcine NLRP3 Inflammasome-Mediated Interleukin-1 Beta Production by Suppressing ASC Ubiquitination.J Virol. 2018 Mar 28;92(8):e00022-18. doi: 10.1128/JVI.00022-18. Print 2018 Apr 15. J Virol. 2018. PMID: 29386291 Free PMC article.
-
The H7N9 influenza A virus infection results in lethal inflammation in the mammalian host via the NLRP3-caspase-1 inflammasome.Sci Rep. 2017 Aug 8;7(1):7625. doi: 10.1038/s41598-017-07384-5. Sci Rep. 2017. PMID: 28790324 Free PMC article.
-
The role of the NLRP3 inflammasome in regulation of antiviral responses to influenza A virus infection.Antiviral Res. 2017 Dec;148:32-42. doi: 10.1016/j.antiviral.2017.10.020. Epub 2017 Oct 31. Antiviral Res. 2017. PMID: 29097227 Review.
-
The RNA- and TRIM25-Binding Domains of Influenza Virus NS1 Protein Are Essential for Suppression of NLRP3 Inflammasome-Mediated Interleukin-1β Secretion.J Virol. 2016 Mar 28;90(8):4105-4114. doi: 10.1128/JVI.00120-16. Print 2016 Apr. J Virol. 2016. PMID: 26865721 Free PMC article.
-
Innate Immune Sensing of Influenza A Virus.Viruses. 2020 Jul 14;12(7):755. doi: 10.3390/v12070755. Viruses. 2020. PMID: 32674269 Free PMC article. Review.
Cited by
-
Pulmonary Toxicity of Polystyrene, Polypropylene, and Polyvinyl Chloride Microplastics in Mice.Molecules. 2022 Nov 16;27(22):7926. doi: 10.3390/molecules27227926. Molecules. 2022. PMID: 36432032 Free PMC article.
-
Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling.J Immunol. 2013 May 1;190(9):4676-84. doi: 10.4049/jimmunol.1202096. Epub 2013 Mar 22. J Immunol. 2013. PMID: 23526820 Free PMC article.
-
IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8⁺ T cell responses to influenza A virus.Nat Immunol. 2013 Mar;14(3):246-53. doi: 10.1038/ni.2514. Epub 2013 Jan 13. Nat Immunol. 2013. PMID: 23314004 Free PMC article.
-
NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases?Cells. 2023 Nov 26;12(23):2709. doi: 10.3390/cells12232709. Cells. 2023. PMID: 38067137 Free PMC article. Review.
-
Inflammasome Genetic Variants Are Associated with Protection to Clinical Severity of COVID-19 among Patients from Rio de Janeiro, Brazil.Biomed Res Int. 2022 Sep 5;2022:9082455. doi: 10.1155/2022/9082455. eCollection 2022. Biomed Res Int. 2022. PMID: 36105941 Free PMC article.
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Cottey R, Rowe CA, Bender BS. Influenza virus. Curr Protoc Immunol . 2001;Chapter 19(Unit 19):11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

