Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart
- PMID: 19237663
- PMCID: PMC2739097
- DOI: 10.1161/CIRCULATIONAHA.108.792101
Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart
Abstract
Background: Diabetes-associated cardiac dysfunction is associated with mitochondrial dysfunction and oxidative stress, which may contribute to left ventricular dysfunction. The contribution of altered myocardial insulin action, independent of associated changes in systemic metabolism, is incompletely understood. The present study tested the hypothesis that perinatal loss of insulin signaling in the heart impairs mitochondrial function.
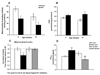
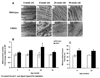
Methods and results: In 8-week-old mice with cardiomyocyte deletion of insulin receptors (CIRKO), inotropic reserves were reduced, and mitochondria manifested respiratory defects for pyruvate that was associated with proportionate reductions in catalytic subunits of pyruvate dehydrogenase. Progressive age-dependent defects in oxygen consumption and ATP synthesis with the substrate glutamate and the fatty acid derivative palmitoyl-carnitine were observed. Mitochondria also were uncoupled when exposed to palmitoyl-carnitine, in part as a result of increased reactive oxygen species production and oxidative stress. Although proteomic and genomic approaches revealed a reduction in subsets of genes and proteins related to oxidative phosphorylation, no reductions in maximal activities of mitochondrial electron transport chain complexes were found. However, a disproportionate reduction in tricarboxylic acid cycle and fatty acid oxidation proteins in mitochondria suggests that defects in fatty acid and pyruvate metabolism and tricarboxylic acid flux may explain the mitochondrial dysfunction observed.
Conclusions: Impaired myocardial insulin signaling promotes oxidative stress and mitochondrial uncoupling, which, together with reduced tricarboxylic acid and fatty acid oxidative capacity, impairs mitochondrial energetics. This study identifies specific contributions of impaired insulin action to mitochondrial dysfunction in the heart.
Conflict of interest statement
(None).
Figures


Similar articles
-
Genetic loss of insulin receptors worsens cardiac efficiency in diabetes.J Mol Cell Cardiol. 2012 May;52(5):1019-26. doi: 10.1016/j.yjmcc.2012.02.001. Epub 2012 Feb 9. J Mol Cell Cardiol. 2012. PMID: 22342406 Free PMC article.
-
Impaired insulin signaling accelerates cardiac mitochondrial dysfunction after myocardial infarction.J Mol Cell Cardiol. 2009 Jun;46(6):910-8. doi: 10.1016/j.yjmcc.2009.02.014. Epub 2009 Feb 26. J Mol Cell Cardiol. 2009. PMID: 19249310 Free PMC article.
-
Mitochondrial Reactive Oxygen Species Mediate Cardiac Structural, Functional, and Mitochondrial Consequences of Diet-Induced Metabolic Heart Disease.J Am Heart Assoc. 2016 Jan 11;5(1):e002555. doi: 10.1161/JAHA.115.002555. J Am Heart Assoc. 2016. PMID: 26755553 Free PMC article.
-
The breathing heart - mitochondrial respiratory chain dysfunction in cardiac disease.Int J Cardiol. 2014 Feb 1;171(2):134-43. doi: 10.1016/j.ijcard.2013.12.014. Epub 2013 Dec 18. Int J Cardiol. 2014. PMID: 24377708 Review.
-
Mitochondrial Bioenergetics and Dysfunction in Failing Heart.Adv Exp Med Biol. 2017;982:65-80. doi: 10.1007/978-3-319-55330-6_4. Adv Exp Med Biol. 2017. PMID: 28551782 Review.
Cited by
-
Stimulation of GLP-1 Receptor Inhibits Methylglyoxal-Induced Mitochondrial Dysfunctions in H9c2 Cardiomyoblasts: Potential Role of Epac/PI3K/Akt Pathway.Front Pharmacol. 2020 May 29;11:805. doi: 10.3389/fphar.2020.00805. eCollection 2020. Front Pharmacol. 2020. PMID: 32547400 Free PMC article.
-
Insulin and Insulin-Like Growth Factor 1 Signaling Preserves Sarcomere Integrity in the Adult Heart.Mol Cell Biol. 2022 Oct 20;42(10):e0016322. doi: 10.1128/mcb.00163-22. Epub 2022 Sep 20. Mol Cell Biol. 2022. PMID: 36125265 Free PMC article.
-
Unbreak my heart: targeting mitochondrial autophagy in diabetic cardiomyopathy.Antioxid Redox Signal. 2015 Jun 10;22(17):1527-44. doi: 10.1089/ars.2015.6322. Epub 2015 Apr 28. Antioxid Redox Signal. 2015. PMID: 25808102 Free PMC article. Review.
-
The absence of insulin signaling in the heart induces changes in potassium channel expression and ventricular repolarization.Am J Physiol Heart Circ Physiol. 2014 Mar 1;306(5):H747-54. doi: 10.1152/ajpheart.00849.2013. Epub 2013 Dec 27. Am J Physiol Heart Circ Physiol. 2014. PMID: 24375641 Free PMC article.
-
Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria.Mol Cell Biochem. 2014 Jan;386(1-2):233-49. doi: 10.1007/s11010-013-1861-x. Epub 2013 Dec 4. Mol Cell Biochem. 2014. PMID: 24307101 Review.
References
-
- Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. - PubMed
-
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. - PubMed
-
- Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. - PubMed
-
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. - PubMed
-
- Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED. Type 1 Diabetic Akita Mouse Hearts are Insulin Sensitive but Manifest Structurally Abnormal Mitochondria that Remain Coupled Despite Increased Uncoupling Protein 3. Diabetes. 2008;57:2924–2932. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK092065/DK/NIDDK NIH HHS/United States
- R01HL070070/HL/NHLBI NIH HHS/United States
- U01 HL087947-03/HL/NHLBI NIH HHS/United States
- R21 DK073590-02/DK/NIDDK NIH HHS/United States
- U01 HL070525-05/HL/NHLBI NIH HHS/United States
- R21 DK073590/DK/NIDDK NIH HHS/United States
- UO1HL70525/HL/NHLBI NIH HHS/United States
- T32 DK091317/DK/NIDDK NIH HHS/United States
- R01 HL070070/HL/NHLBI NIH HHS/United States
- UO1HL087947/HL/NHLBI NIH HHS/United States
- R21DK073590/DK/NIDDK NIH HHS/United States
- R01 HL070070-04/HL/NHLBI NIH HHS/United States
- U01 HL087947/HL/NHLBI NIH HHS/United States
- U01 HL070525/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

