CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche
- PMID: 19228948
- PMCID: PMC2656176
- DOI: 10.1073/pnas.0809736106
CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche
Abstract
Plasma cells represent the end stage of B-cell development and play a key role in providing an efficient antibody response, but they are also involved in numerous pathologies. Here we show that CD93, a receptor expressed during early B-cell development, is reinduced during plasma-cell differentiation. High CD93/CD138 expression was restricted to antibody-secreting cells both in T-dependent and T-independent responses as naive, memory, and germinal-center B cells remained CD93-negative. CD93 was expressed on (pre)plasmablasts/plasma cells, including long-lived plasma cells that showed decreased cell cycle activity, high levels of isotype-switched Ig secretion, and modification of the transcriptional network. T-independent and T-dependent stimuli led to re-expression of CD93 via 2 pathways, either before or after CD138 or Blimp-1 expression. Strikingly, while humoral immune responses initially proceeded normally, CD93-deficient mice were unable to maintain antibody secretion and bone-marrow plasma-cell numbers, demonstrating that CD93 is important for the maintenance of plasma cells in bone marrow niches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


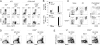
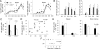
Similar articles
-
Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo.J Immunol. 2000 Nov 15;165(10):5462-71. doi: 10.4049/jimmunol.165.10.5462. J Immunol. 2000. PMID: 11067898
-
Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1.J Exp Med. 2005 Mar 21;201(6):993-1005. doi: 10.1084/jem.20042239. Epub 2005 Mar 14. J Exp Med. 2005. PMID: 15767369 Free PMC article.
-
Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow.J Exp Med. 2005 Dec 5;202(11):1471-6. doi: 10.1084/jem.20051611. Epub 2005 Nov 28. J Exp Med. 2005. PMID: 16314438 Free PMC article.
-
Limited humoral immunoglobulin E memory influences serum immunoglobulin E levels in blood.Clin Exp Allergy. 2009 Sep;39(9):1307-13. doi: 10.1111/j.1365-2222.2009.03278.x. Epub 2009 May 20. Clin Exp Allergy. 2009. PMID: 19489847 Free PMC article. Review.
-
Regulatory mechanisms that determine the development and function of plasma cells.Annu Rev Immunol. 2003;21:205-30. doi: 10.1146/annurev.immunol.21.120601.141138. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12524387 Review.
Cited by
-
Immunophenotyping of the Medullary B Cell Compartment In Mouse Models.Methods Mol Biol. 2021;2308:95-105. doi: 10.1007/978-1-0716-1425-9_8. Methods Mol Biol. 2021. PMID: 34057717
-
New insights into the ontogeny, diversity, maturation and survival of long-lived plasma cells.Nat Rev Immunol. 2024 Jul;24(7):461-470. doi: 10.1038/s41577-024-00991-0. Epub 2024 Feb 8. Nat Rev Immunol. 2024. PMID: 38332373 Review.
-
Unraveling the diversity and functions of tissue-resident plasma cells.Nat Immunol. 2024 Feb;25(2):330-342. doi: 10.1038/s41590-023-01712-w. Epub 2024 Jan 3. Nat Immunol. 2024. PMID: 38172260
-
Defective LAT signalosome pathology in mice mimics human IgG4-related disease at single-cell level.J Exp Med. 2023 Nov 6;220(11):e20231028. doi: 10.1084/jem.20231028. Epub 2023 Aug 25. J Exp Med. 2023. PMID: 37624388 Free PMC article.
-
Role of CD93 in Health and Disease.Cells. 2023 Jul 4;12(13):1778. doi: 10.3390/cells12131778. Cells. 2023. PMID: 37443812 Free PMC article. Review.
References
-
- Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. - PubMed
-
- Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. - PubMed
-
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. - PubMed
-
- Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

