Kinesin-8 from fission yeast: a heterodimeric, plus-end-directed motor that can couple microtubule depolymerization to cargo movement
- PMID: 19037096
- PMCID: PMC2633396
- DOI: 10.1091/mbc.e08-09-0979
Kinesin-8 from fission yeast: a heterodimeric, plus-end-directed motor that can couple microtubule depolymerization to cargo movement
Abstract
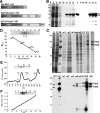
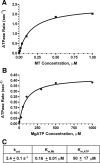
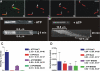
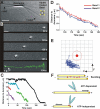
Fission yeast expresses two kinesin-8s, previously identified and characterized as products of the klp5(+) and klp6(+) genes. These polypeptides colocalize throughout the vegetative cell cycle as they bind cytoplasmic microtubules during interphase, spindle microtubules, and/or kinetochores during early mitosis, and the interpolar spindle as it elongates in anaphase B. Here, we describe in vitro properties of these motor proteins and some truncated versions expressed in either bacteria or Sf9 cells. The motor-plus-neck domain of Klp6p formed soluble dimers that cross-linked microtubules and showed both microtubule-activated ATPase and plus-end-directed motor activities. Full-length Klp5p and Klp6p, coexpressed in Sf9 cells, formed soluble heterodimers with the same activities. The latter recombinant protein could also couple microbeads to the ends of shortening microtubules and use energy from tubulin depolymerization to pull a load in the minus end direction. These results, together with the spindle localizations of these proteins in vivo and their requirement for cell viability in the absence of the Dam1/DASH kinetochore complex, support the hypothesis that fission yeast kinesin-8 contributes both to chromosome congression to the metaphase plate and to the coupling of spindle microtubules to kinetochores during anaphase A.
Figures

Similar articles
-
Novel interactions of fission yeast kinesin 8 revealed through in vivo expression of truncation alleles.Cell Motil Cytoskeleton. 2008 Aug;65(8):626-40. doi: 10.1002/cm.20289. Cell Motil Cytoskeleton. 2008. PMID: 18553361 Free PMC article.
-
Metaphase kinetochore movements are regulated by kinesin-8 motors and microtubule dynamic instability.Mol Biol Cell. 2018 Jun 1;29(11):1332-1345. doi: 10.1091/mbc.E17-11-0667. Epub 2018 Apr 5. Mol Biol Cell. 2018. PMID: 29851559 Free PMC article.
-
Kinesin-8 and Dis1/TOG collaborate to limit spindle elongation from prophase to anaphase A for proper chromosome segregation in fission yeast.J Cell Sci. 2019 Sep 23;132(18):jcs232306. doi: 10.1242/jcs.232306. J Cell Sci. 2019. PMID: 31427431 Free PMC article.
-
CLIP-170 family members: a motor-driven ride to microtubule plus ends.Dev Cell. 2004 Jun;6(6):746-8. doi: 10.1016/j.devcel.2004.05.017. Dev Cell. 2004. PMID: 15177023 Review.
-
Kinesin-8 molecular motors: putting the brakes on chromosome oscillations.Trends Cell Biol. 2008 Jul;18(7):307-10. doi: 10.1016/j.tcb.2008.05.003. Epub 2008 May 29. Trends Cell Biol. 2008. PMID: 18513970 Free PMC article. Review.
Cited by
-
Inositol Pyrophosphate Kinase Asp1 Modulates Chromosome Segregation Fidelity and Spindle Function in Schizosaccharomyces pombe.Mol Cell Biol. 2016 Nov 28;36(24):3128-3140. doi: 10.1128/MCB.00330-16. Print 2016 Dec 15. Mol Cell Biol. 2016. PMID: 27697865 Free PMC article.
-
Tubulin depolymerization may be an ancient biological motor.J Cell Sci. 2010 Oct 15;123(Pt 20):3425-34. doi: 10.1242/jcs.067611. J Cell Sci. 2010. PMID: 20930138 Free PMC article. Review.
-
Toward a comprehensive and quantitative understanding of intracellular microtubule organization.Mol Syst Biol. 2009;5:251. doi: 10.1038/msb.2009.7. Epub 2009 Mar 17. Mol Syst Biol. 2009. PMID: 19293831 Free PMC article. No abstract available.
-
Chiasmata and the kinetochore component Dam1 are crucial for elimination of erroneous chromosome attachments and centromere oscillation at meiosis I.Open Biol. 2021 Feb;11(2):200308. doi: 10.1098/rsob.200308. Epub 2021 Feb 3. Open Biol. 2021. PMID: 33529549 Free PMC article.
-
Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters.Cell. 2012 Feb 3;148(3):502-14. doi: 10.1016/j.cell.2012.01.007. Cell. 2012. PMID: 22304918 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

