Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate
- PMID: 19019830
- PMCID: PMC2615505
- DOI: 10.1074/jbc.M807270200
Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate
Abstract
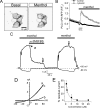
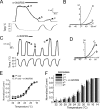
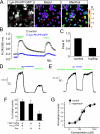
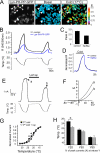
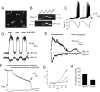
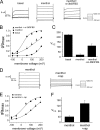
Cold temperatures robustly activate a small cohort of somatosensory nerves, yet during a prolonged cold stimulus their activity will decrease, or adapt, over time. This process allows for the discrimination of subtle changes in temperature. At the molecular level, cold is detected by transient receptor potential melastatin 8 (TRPM8), a nonselective cation channel expressed on a subset of peripheral afferent fibers. We and others have reported that TRPM8 channels also adapt in a calcium-dependent manner when activated by the cooling compound menthol. Additionally, TRPM8 activity is sensitive to the phospholipid phosphoinositol 4,5-bisphosphate (PIP2), a substrate for the enzyme phospholipase C (PLC). These results suggest an adaptation model whereby TRPM8-mediated Ca2+ influx activates PLC, thereby decreasing PIP2 levels and resulting in reduced TRPM8 activity. Here we tested this model using pharmacological activation of PLC and by manipulating PIP2 levels independent of both PLC and Ca2+. PLC activation leads to adaptation-like reductions in cold- or menthol-evoked TRPM8 currents in both heterologous and native cells. Moreover, PLC-independent reductions in PIP2 had a similar effect on cold- and menthol-evoked currents. Mechanistically, either form of adaptation does not alter temperature sensitivity of TRPM8 but does lead to a change in channel gating. Our results show that adaptation is a shift in voltage dependence toward more positive potentials, reversing the trend toward negative potentials caused by agonist. These data suggest that PLC activity not only mediates adaptation to thermal stimuli, but likely underlies a more general mechanism that establishes the temperature sensitivity of somatosensory neurons.
Figures


Similar articles
-
Phospholipase C δ4 regulates cold sensitivity in mice.J Physiol. 2016 Jul 1;594(13):3609-28. doi: 10.1113/JP272321. Epub 2016 May 29. J Physiol. 2016. PMID: 27062607 Free PMC article.
-
Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels.J Physiol. 2011 Dec 15;589(Pt 24):6007-27. doi: 10.1113/jphysiol.2011.220228. Epub 2011 Oct 17. J Physiol. 2011. PMID: 22005680 Free PMC article.
-
Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives.J Biol Chem. 2009 Feb 13;284(7):4102-11. doi: 10.1074/jbc.M806651200. Epub 2008 Dec 18. J Biol Chem. 2009. PMID: 19095656
-
Regulation of the cold-sensing TRPM8 channels by phosphoinositides and Gq-coupled receptors.Channels (Austin). 2020 Dec;14(1):79-86. doi: 10.1080/19336950.2020.1734266. Channels (Austin). 2020. PMID: 32101066 Free PMC article. Review.
-
Regulation of TRPM8 channel activity.Mol Cell Endocrinol. 2012 Apr 28;353(1-2):68-74. doi: 10.1016/j.mce.2011.10.023. Epub 2011 Oct 28. Mol Cell Endocrinol. 2012. PMID: 22061619 Free PMC article. Review.
Cited by
-
Molecular basis of peripheral innocuous cold sensitivity.Handb Clin Neurol. 2018;156:57-67. doi: 10.1016/B978-0-444-63912-7.00003-5. Handb Clin Neurol. 2018. PMID: 30454609 Free PMC article. Review.
-
Smelling the difference: controversial ideas in insect olfaction.J Exp Biol. 2009 Jul;212(Pt 13):1973-9. doi: 10.1242/jeb.023036. J Exp Biol. 2009. PMID: 19525421 Free PMC article.
-
Cold-inducible RNA-binding protein migrates from the nucleus to the cytoplasm under cold stress in normal human bronchial epithelial cells via TRPM8-mediated mechanism.Ann Transl Med. 2021 Sep;9(18):1470. doi: 10.21037/atm-21-4447. Ann Transl Med. 2021. PMID: 34734022 Free PMC article.
-
Chronic morphine regulates TRPM8 channels via MOR-PKCβ signaling.Mol Brain. 2020 Apr 14;13(1):61. doi: 10.1186/s13041-020-00599-0. Mol Brain. 2020. PMID: 32290846 Free PMC article.
-
Regulation of thermoTRPs by lipids.Temperature (Austin). 2016 Nov 1;4(1):24-40. doi: 10.1080/23328940.2016.1254136. eCollection 2017. Temperature (Austin). 2016. PMID: 28349093 Free PMC article. Review.
References
-
- Julius, D., and Basbaum, A. I. (2001) Nature 413 203-210 - PubMed
-
- Byers, M. R., and Narhi, M. V. O. (2002) in Dental Pulp (Hargreaves, K. M., and Goodis, H. E., eds) Vol. 3, pp. 151-179, Quintessence Publishing Co, Inc, Carol Stream, IL
-
- Han, Z. S., Zhang, E. T., and Craig, A. D. (1998) Nat. Neurosci. 1 218-225 - PubMed
-
- Jordt, S. E., McKemy, D. D., and Julius, D. (2003) Curr. Opin. Neurobiol. 13 487-492 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

