Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax
- PMID: 18981409
- PMCID: PMC2577705
- DOI: 10.1073/pnas.0808691105
Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax
Erratum in
- Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1678
Abstract
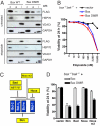
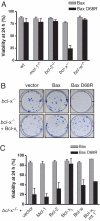
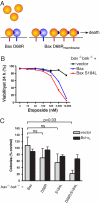
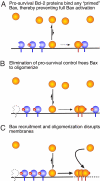
A central issue in the control of apoptosis is whether its essential mediators Bax and Bak must be restrained by Bcl-2-like prosurvival relatives to prevent their damaging mitochondria and unleashing apoptosis. The issue is particularly vexed for Bax, which is largely a cytosolic monomer in unstressed cells. To determine whether Bax regulation requires its binding by prosurvival relatives, we replaced a conserved aspartate in its BH3 interaction domain with arginine. Bax D68R functioned and behaved like wild-type Bax in localization and activation but had greatly impaired binding to the prosurvival family members. Nevertheless, Bcl-x(L) remained able to block apoptosis induced by Bax D68R. Whereas cells with sufficient Bcl-x(L) tolerated expression of Bax D68R, it provoked apoptosis when Bcl-x(L) was absent, downregulated, or inactivated. Moreover, Bax D68R rendered membrane bound by a C-terminal anchor mutation overwhelmed endogenous Bcl-x(L) and killed cells. These unexpected results suggest that engagement of Bax by its prosurvival relatives is a major barrier to its full activation. We propose that the Bcl-2-like proteins must capture the small proportion of Bax molecules with an exposed BH3 domain, probably on the mitochondrial membrane, to prevent Bax-imposed cell death, but that Bcl-x(L) also controls Bax by other mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Targeting Bax interaction sites reveals that only homo-oligomerization sites are essential for its activation.Cell Death Differ. 2013 May;20(5):744-54. doi: 10.1038/cdd.2013.4. Epub 2013 Feb 8. Cell Death Differ. 2013. PMID: 23392123 Free PMC article.
-
Characterization of the anti-apoptotic mechanism of Bcl-B.Biochem J. 2003 Nov 15;376(Pt 1):229-36. doi: 10.1042/BJ20030374. Biochem J. 2003. PMID: 12921534 Free PMC article.
-
Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins.Genes Dev. 2005 Jun 1;19(11):1294-305. doi: 10.1101/gad.1304105. Epub 2005 May 18. Genes Dev. 2005. PMID: 15901672 Free PMC article.
-
How the Bcl-2 family of proteins interact to regulate apoptosis.Cell Res. 2006 Feb;16(2):203-13. doi: 10.1038/sj.cr.7310028. Cell Res. 2006. PMID: 16474435 Review.
-
Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): keep your friends close but your enemies closer.Int J Biochem Cell Biol. 2013 Jan;45(1):64-7. doi: 10.1016/j.biocel.2012.09.022. Epub 2012 Oct 11. Int J Biochem Cell Biol. 2013. PMID: 23064052 Review.
Cited by
-
BAX insertion, oligomerization, and outer membrane permeabilization in brain mitochondria: role of permeability transition and SH-redox regulation.Biochim Biophys Acta. 2010 Nov;1797(11):1795-806. doi: 10.1016/j.bbabio.2010.07.006. Epub 2010 Jul 23. Biochim Biophys Acta. 2010. PMID: 20655869 Free PMC article.
-
Structural basis for apoptosis inhibition by Epstein-Barr virus BHRF1.PLoS Pathog. 2010 Dec 23;6(12):e1001236. doi: 10.1371/journal.ppat.1001236. PLoS Pathog. 2010. PMID: 21203485 Free PMC article.
-
Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis.Cell Death Differ. 2014 Feb;21(2):196-205. doi: 10.1038/cdd.2013.139. Epub 2013 Oct 25. Cell Death Differ. 2014. PMID: 24162660 Free PMC article. Review.
-
Robust autoactivation for apoptosis by BAK but not BAX highlights BAK as an important therapeutic target.Cell Death Dis. 2020 Apr 23;11(4):268. doi: 10.1038/s41419-020-2463-7. Cell Death Dis. 2020. PMID: 32327636 Free PMC article.
-
Significance of p53 dynamics in regulating apoptosis in response to ionizing radiation, and polypharmacological strategies.Sci Rep. 2014 Sep 1;4:6245. doi: 10.1038/srep06245. Sci Rep. 2014. PMID: 25175563 Free PMC article.
References
-
- Youle RJ, Strasser A. The Bcl-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
-
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. - PubMed
-
- Hsu Y-T, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

