Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2
- PMID: 18784258
- PMCID: PMC2584909
- DOI: 10.1152/ajprenal.90445.2008
Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2
Abstract
Homozygous mice carrying kd (kidney disease) mutations in the gene encoding prenyl diphosphate synthase subunit 2 (Pdss2kd/kd) develop interstitial nephritis and eventually die from end-stage renal disease. The PDSS2 polypeptide in concert with PDSS1 synthesizes the polyisoprenyl tail of coenzyme Q (Q or ubiquinone), a lipid quinone required for mitochondrial respiratory electron transport. We have shown that a deficiency in Q content is evident in Pdss2kd/kd mouse kidney lipid extracts by 40 days of age and thus precedes the onset of proteinuria and kidney disease by several weeks. The presence of the kd (V117M) mutation in PDSS2 does not prevent its association with PDSS1. However, heterologous expression of the kd mutant form of PDSS2 together with PDSS1 in Escherichia coli recapitulates the Q deficiency observed in the Pdss2kd/kd mouse. Dietary supplementation with Q10 provides a dramatic rescue of both proteinuria and interstitial nephritis in the Pdss2kd/kd mutant mice. The results presented suggest that Q may be acting as a potent lipid-soluble antioxidant, rather than by boosting kidney mitochondrial respiration. Such Q10 supplementation may have profound and beneficial effects in treatment of certain forms of focal segmental glomerulosclerosis that mirror the renal disease of the Pdss2kd/kd mouse.
Figures

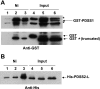
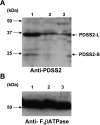

Similar articles
-
Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease.PLoS Genet. 2008 Apr 25;4(4):e1000061. doi: 10.1371/journal.pgen.1000061. PLoS Genet. 2008. PMID: 18437205 Free PMC article.
-
Focal segmental glomerulosclerosis is associated with a PDSS2 haplotype and, independently, with a decreased content of coenzyme Q10.Am J Physiol Renal Physiol. 2013 Oct 15;305(8):F1228-38. doi: 10.1152/ajprenal.00143.2013. Epub 2013 Aug 7. Am J Physiol Renal Physiol. 2013. PMID: 23926186 Free PMC article.
-
Tissue-specific oxidative stress and loss of mitochondria in CoQ-deficient Pdss2 mutant mice.FASEB J. 2013 Feb;27(2):612-21. doi: 10.1096/fj.12-209361. Epub 2012 Nov 12. FASEB J. 2013. PMID: 23150520 Free PMC article.
-
Coenzyme Q10 supplementation therapy for 2 children with proteinuria renal disease and ADCK4 mutation: Case reports and literature review.Medicine (Baltimore). 2017 Nov;96(47):e8880. doi: 10.1097/MD.0000000000008880. Medicine (Baltimore). 2017. PMID: 29382012 Free PMC article. Review.
-
[Progress in mitochondrial nephropathy].Zhonghua Er Ke Za Zhi. 2014 Jul;52(7):503-5. Zhonghua Er Ke Za Zhi. 2014. PMID: 25224054 Review. Chinese. No abstract available.
Cited by
-
Expression of the human atypical kinase ADCK3 rescues coenzyme Q biosynthesis and phosphorylation of Coq polypeptides in yeast coq8 mutants.Biochim Biophys Acta. 2011 May;1811(5):348-60. doi: 10.1016/j.bbalip.2011.01.009. Epub 2011 Feb 4. Biochim Biophys Acta. 2011. PMID: 21296186 Free PMC article.
-
Nutritional Interventions for Mitochondrial OXPHOS Deficiencies: Mechanisms and Model Systems.Annu Rev Pathol. 2018 Jan 24;13:163-191. doi: 10.1146/annurev-pathol-020117-043644. Epub 2017 Nov 3. Annu Rev Pathol. 2018. PMID: 29099651 Free PMC article. Review.
-
Mitochondrial Disease and the Kidney With a Special Focus on CoQ10 Deficiency.Kidney Int Rep. 2020 Oct 10;5(12):2146-2159. doi: 10.1016/j.ekir.2020.09.044. eCollection 2020 Dec. Kidney Int Rep. 2020. PMID: 33305107 Free PMC article. Review.
-
Mitochondrial dysfunction in inherited renal disease and acute kidney injury.Nat Rev Nephrol. 2016 May;12(5):267-80. doi: 10.1038/nrneph.2015.214. Epub 2016 Jan 25. Nat Rev Nephrol. 2016. PMID: 26804019 Free PMC article. Review.
-
Redefining infantile-onset multisystem phenotypes of coenzyme Q10-deficiency in the next-generation sequencing era.J Transl Genet Genom. 2020;4:22-35. doi: 10.20517/jtgg.2020.02. Epub 2020 Apr 23. J Transl Genet Genom. 2020. PMID: 33426503 Free PMC article.
References
-
- Barisoni L, Diomedi-Camassei F, Santorelli FM, Caridi G, Thomas DB, Emma F, Piemonte F, Ghiggeri GM. Collapsing glomerulopathy associated with inherited mitochondrial injury. Kidney Int 74: 237–243, 2008. - PubMed
-
- Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion 7S: S41–S50, 2007. - PubMed
-
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet 8: 835–844, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

