Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence
- PMID: 18753206
- PMCID: PMC2573162
- DOI: 10.1128/JVI.00752-08
Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence
Abstract
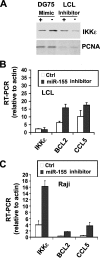
MicroRNAs have been implicated in the modulation of gene expression programs important for normal and cancer cell development. miR-155 is known to play a role in B-cell development and is upregulated in various B-cell lymphomas, including several that are latently infected with Epstein-Barr virus (EBV). We show here that EBV infection of primary human B lymphocytes leads to the sustained elevation of miR-155 and its precursor RNA, BIC. The EBV-encoded latency membrane protein 1 (LMP1) can partially reconstitute BIC activation in B lymphocytes but not in epithelial cell cultures. LMP1 is a potent activator of NF-kappaB signaling pathways and is essential for EBV immortalization of B lymphocytes. An inhibitor to miR-155 further stimulated NF-kappaB responsive gene transcription, and IKKepsilon was identified as a potential target of miR-155 translational repression. Remarkably, miR-155 inhibitor reduced EBNA1 mRNA and the EBV copy number in latently infected cells. This suggests that miR-155 contributes to EBV immortalization by modulation of NF-kappaB signaling and the suppression of host innate immunity to latent viral infection.
Figures
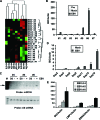
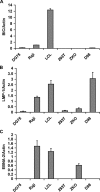
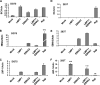
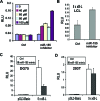
Similar articles
-
Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-kappaB pathway.Nucleic Acids Res. 2008 Nov;36(20):6608-19. doi: 10.1093/nar/gkn666. Epub 2008 Oct 21. Nucleic Acids Res. 2008. PMID: 18940871 Free PMC article.
-
Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a.RNA Biol. 2007 Nov;4(3):131-7. doi: 10.4161/rna.4.3.5206. RNA Biol. 2007. PMID: 18347435
-
Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells.J Virol. 2004 Apr;78(8):4108-19. doi: 10.1128/jvi.78.8.4108-4119.2004. J Virol. 2004. PMID: 15047827 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
EBV-Encoded Latent Genes.Adv Exp Med Biol. 2018;1045:377-394. doi: 10.1007/978-981-10-7230-7_17. Adv Exp Med Biol. 2018. PMID: 29896676 Review.
Cited by
-
Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10276-81. doi: 10.1073/pnas.1303603110. Epub 2013 Jun 3. Proc Natl Acad Sci U S A. 2013. PMID: 23733960 Free PMC article.
-
The Epstein-Barr virus (EBV)-induced tumor suppressor microRNA MiR-34a is growth promoting in EBV-infected B cells.J Virol. 2012 Jun;86(12):6889-98. doi: 10.1128/JVI.07056-11. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496226 Free PMC article.
-
Alteration of microRNA profiles in squamous cell carcinoma of the head and neck cell lines by human papillomavirus.Head Neck. 2011 Apr;33(4):504-12. doi: 10.1002/hed.21475. Head Neck. 2011. PMID: 20652977 Free PMC article.
-
MicroRNAs of the immune system: roles in inflammation and cancer.Ann N Y Acad Sci. 2010 Jan;1183:183-94. doi: 10.1111/j.1749-6632.2009.05121.x. Ann N Y Acad Sci. 2010. PMID: 20146715 Free PMC article. Review.
-
Modulation of Angiogenic Processes by the Human Gammaherpesviruses, Epstein-Barr Virus and Kaposi's Sarcoma-Associated Herpesvirus.Front Microbiol. 2019 Jul 12;10:1544. doi: 10.3389/fmicb.2019.01544. eCollection 2019. Front Microbiol. 2019. PMID: 31354653 Free PMC article. Review.
References
-
- Agami, R. 2007. All roads lead to IKKɛ. Cell 1291043-1045. - PubMed
-
- Calame, K. 2007. microRNA-155 function in B cells. Immunity 27825-827. - PubMed
-
- Cullen, B. R. 2006. Viruses and microRNAs. Nat. Genet. 38(Suppl.)S25-S30. - PubMed
-
- Dirmeier, U., R. Hoffmann, E. Kilger, U. Schultheiss, C. Briseno, O. Gires, A. Kieser, D. Eick, B. Sugden, and W. Hammerschmidt. 2005. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene 241711-1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

