NF-kappaB signaling differentially regulates influenza virus RNA synthesis
- PMID: 18701591
- PMCID: PMC2566266
- DOI: 10.1128/JVI.00909-08
NF-kappaB signaling differentially regulates influenza virus RNA synthesis
Abstract
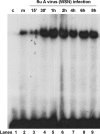
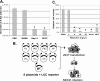
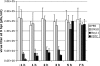
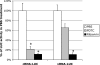
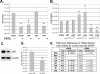
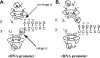
The NF-kappaB signaling pathway has previously been shown to be required for efficient influenza A virus replication, although the molecular mechanism is not well understood. In this study, we identified a specific step of the influenza virus life cycle that is influenced by NF-kappaB signaling by using two known NF-kappaB inhibitors and a variety of influenza virus-specific assays. The results of time course experiments suggest that the NF-kappaB inhibitors Bay11-7082 and ammonium pyrrolidinedithiocarbamate inhibited an early postentry step of viral infection, but they did not appear to affect the nucleocytoplasmic trafficking of the viral ribonucleoprotein complex. Instead, we found that the levels of influenza virus genomic RNA (vRNA), but not the corresponding cRNA or mRNA, were specifically reduced by the inhibitors in virus-infected cells, indicating that NF-kappaB signaling is intimately involved in the vRNA synthesis. Furthermore, we showed that the NF-kappaB inhibitors specifically diminished influenza virus RNA transcription from the cRNA promoter but not from the vRNA promoter in a reporter assay, a result which is consistent with data obtained from virus-infected cells. The overexpression of the p65 NF-kappaB molecule could not only eliminate the inhibition but also activate influenza virus RNA transcription from the cRNA promoter. Finally, using p65-specific small interfering RNA, we have shown that p65 knockdown reduced the levels of influenza virus replication and vRNA synthesis. In summary, we have provided evidence showing, for the first time, that the NF-kappaB host signaling pathway can differentially regulate influenza virus RNA synthesis, which may also offer some new perspectives into understanding the host regulation of RNA synthesis by other RNA viruses.
Figures

Similar articles
-
The Host Factor ANP32A Is Required for Influenza A Virus vRNA and cRNA Synthesis.J Virol. 2022 Feb 23;96(4):e0209221. doi: 10.1128/jvi.02092-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935435 Free PMC article.
-
Temperature sensitive influenza A virus genome replication results from low thermal stability of polymerase-cRNA complexes.Virol J. 2006 Aug 25;3:58. doi: 10.1186/1743-422X-3-58. Virol J. 2006. PMID: 16934156 Free PMC article.
-
Mutation of an Influenza Virus Polymerase 3' RNA Promoter Binding Site Inhibits Transcription Elongation.J Virol. 2020 Jun 16;94(13):e00498-20. doi: 10.1128/JVI.00498-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295915 Free PMC article.
-
The RNA polymerase of influenza a virus: mechanisms of viral transcription and replication.Acta Virol. 2013;57(2):113-22. doi: 10.4149/av_2013_02_113. Acta Virol. 2013. PMID: 23600869 Review.
-
Transcription and replication of the influenza a virus genome.Acta Virol. 2000 Oct;44(5):273-82. Acta Virol. 2000. PMID: 11252672 Review.
Cited by
-
Clinical Outcomes of Early Treatment With Doxycycline for 89 High-Risk COVID-19 Patients in Long-Term Care Facilities in New York.Cureus. 2020 Aug 11;12(8):e9658. doi: 10.7759/cureus.9658. Cureus. 2020. PMID: 32802622 Free PMC article.
-
Mechanisms of geomagnetic field influence on gene expression using influenza as a model system: basics of physical epidemiology.Int J Environ Res Public Health. 2010 Mar;7(3):938-65. doi: 10.3390/ijerph7030938. Epub 2010 Mar 10. Int J Environ Res Public Health. 2010. PMID: 20617011 Free PMC article.
-
The intricate interplay between RNA viruses and NF-κB.Biochim Biophys Acta. 2014 Nov;1843(11):2754-2764. doi: 10.1016/j.bbamcr.2014.08.004. Epub 2014 Aug 10. Biochim Biophys Acta. 2014. PMID: 25116307 Free PMC article. Review.
-
MicroRNA-302a suppresses influenza A virus-stimulated interferon regulatory factor-5 expression and cytokine storm induction.J Biol Chem. 2017 Dec 29;292(52):21291-21303. doi: 10.1074/jbc.M117.805937. Epub 2017 Oct 18. J Biol Chem. 2017. PMID: 29046356 Free PMC article.
-
ROCK1/MLC2 inhibition induces decay of viral mRNA in BPXV infected cells.Sci Rep. 2022 Oct 24;12(1):17811. doi: 10.1038/s41598-022-21610-9. Sci Rep. 2022. PMID: 36280692 Free PMC article.
References
-
- Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25280-288. - PubMed
-
- Brinkmann, M. M., and T. F. Schulz. 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J. Gen. Virol. 871047-1074. - PubMed
-
- Dejardin, E., N. M. Droin, M. Delhase, E. Haas, Y. Cao, C. Makris, Z. W. Li, M. Karin, C. F. Ware, and D. R. Green. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17525-535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

