Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation
- PMID: 18567579
- PMCID: PMC2528995
- DOI: 10.1074/jbc.M800434200
Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation
Abstract
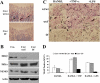
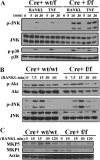
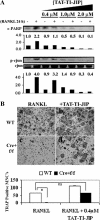
It has been reported previously that inhibitory kappaB kinase (IKK) supports osteoclastogenesis through NF-kappaB-mediated prevention of apoptosis. This finding suggests that the ligand for receptor activator of NF-kappaB (RANKL), the master osteoclastogenic cytokine, induces apoptosis of osteoclast precursors (OCPs) in the absence of IKKbeta/NF-kappaB competency. To validate this hypothesis, we sought to determine the pro-apoptotic signaling factors induced by RANKL in IKKbeta-null osteoclast OCPs and to rescue osteoclast differentiation in the absence of IKKbeta through their inhibition. To accomplish this, we generated mice that lack IKKbeta in multiple hematopoietic lineages, including OCPs. We found that these mice possess both in vitro and in vivo defects in osteoclast generation, in concurrence with previous reports, and that this defect is a result of susceptibility to RANKL-mediated apoptosis as a result of gain-of-function of JNK activation. We demonstrate that differentiation of OCPs depends on IKKbeta because reduced IKKbeta mRNA expression correlates with impaired induction of osteoclast differentiation markers in response to RANKL stimulation. We further show that fine-tuned inhibition of JNK activation in these cells inhibits RANKL-induced apoptosis and restores the ability of IKKbeta-null OCPs to become mature osteoclasts. Our data highlight the pro-osteoclastogenic and anti-apoptotic roles of IKKbeta in OCPs and identify a pro-apoptotic mechanism activated within the RANK signalosome.
Figures
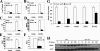


Similar articles
-
Osteoclast differentiation is impaired in the absence of inhibitor of kappa B kinase alpha.J Biol Chem. 2004 Dec 24;279(52):54841-8. doi: 10.1074/jbc.M406392200. Epub 2004 Oct 14. J Biol Chem. 2004. PMID: 15485831
-
Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis.J Immunol. 2004 May 15;172(10):5940-7. doi: 10.4049/jimmunol.172.10.5940. J Immunol. 2004. PMID: 15128775
-
NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis.J Bone Miner Res. 2002 Jul;17(7):1200-10. doi: 10.1359/jbmr.2002.17.7.1200. J Bone Miner Res. 2002. PMID: 12096833
-
Osteoclast differentiation by RANKL requires NF-kappaB-mediated downregulation of cyclin-dependent kinase 6 (Cdk6).J Bone Miner Res. 2004 Jul;19(7):1128-36. doi: 10.1359/jbmr.2004.19.7.1128. Epub 2004 May 24. J Bone Miner Res. 2004. PMID: 15176996
-
Intestinal M cells: Tireless samplers of enteric microbiota.Traffic. 2020 Jan;21(1):34-44. doi: 10.1111/tra.12707. Epub 2019 Nov 28. Traffic. 2020. PMID: 31647148 Review.
Cited by
-
Oxyresveratrol attenuates bone resorption by inhibiting the mitogen-activated protein kinase pathway in ovariectomized rats.Nutr Metab (Lond). 2024 Jan 19;21(1):7. doi: 10.1186/s12986-024-00781-4. Nutr Metab (Lond). 2024. PMID: 38243227 Free PMC article.
-
The Role of NF-κB in Physiological Bone Development and Inflammatory Bone Diseases: Is NF-κB Inhibition "Killing Two Birds with One Stone"?Cells. 2019 Dec 14;8(12):1636. doi: 10.3390/cells8121636. Cells. 2019. PMID: 31847314 Free PMC article. Review.
-
Targeting the NF-κB signaling pathway in chronic tendon disease.Sci Transl Med. 2019 Feb 27;11(481):eaav4319. doi: 10.1126/scitranslmed.aav4319. Sci Transl Med. 2019. PMID: 30814338 Free PMC article.
-
NIK stabilization in osteoclasts results in osteoporosis and enhanced inflammatory osteolysis.PLoS One. 2010 Nov 8;5(11):e15383. doi: 10.1371/journal.pone.0015383. PLoS One. 2010. PMID: 21151480 Free PMC article.
-
The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice.Br J Pharmacol. 2012 Jun;166(3):1084-96. doi: 10.1111/j.1476-5381.2012.01856.x. Br J Pharmacol. 2012. PMID: 22250848 Free PMC article.
References
-
- Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S., Oliveira-dos-Santos, A. J., Van, G., Itie, A., Khoo, W., Wakeham, A., Dunstan, C. R., Lacey, D. L., Mak, T. W., Boyle, W. J., and Penninger, J. M. (1999) Nature 397 315–323 - PubMed
-
- Yoshida, H., Hayashi, S.-I., Kunisada, T., Ogawa, M., Nishikawa, S., Okamura, H., Sudo, T., Shultz, L. D., and Nishikawa, S.-I. (1990) Nature 345 442– 443 - PubMed
-
- Anderson, D. M., Maraskovsky, E., Billingsley, W. L., Dougall, W. C., Tometsko, M. E., Roux, E. R., Teepe, M. C., DuBose, R. F., Cosman, D., and Galibert, L. (1997) Nature 390 175–179 - PubMed
-
- Iotsova, V., Caamano, J., Loy, J., Young, Y., Lewin, A., and Bravo, R. (1997) Nat. Med. 3 1285–1289 - PubMed
-
- Yamashita, T., Yao, Z., Li, F., Zhang, Q., Badell, I. R., Schwarz, E. M., Takeshita, S., Wagner, E. F., Noda, M., Matsuo, K., Xing, L., and Boyce, B. F. (2007) J. Biol. Chem. 282 18245–18253 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

