Kaposi's sarcoma-associated herpesvirus confers a survival advantage to endothelial cells
- PMID: 18559509
- PMCID: PMC2612117
- DOI: 10.1158/0008-5472.CAN-07-5988
Kaposi's sarcoma-associated herpesvirus confers a survival advantage to endothelial cells
Abstract
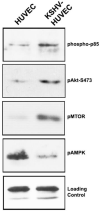
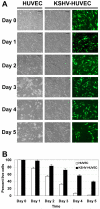
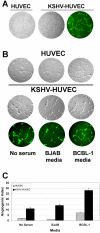
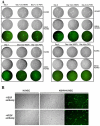
Kaposi's sarcoma-associated herpesvirus (KSHV) is associated with three different human malignancies, including Kaposi's sarcoma (KS), primary effusion lymphoma, and multicentric Castleman's disease. The KS lesion is of endothelial cell in origin and is highly dependent on autocrine and paracrine factors for survival and growth. In this study, we show that KSHV infection of endothelial cells induces the activation of the prosurvival phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin pathway. KSHV infection of endothelial cells augmented cell survival in the presence of apoptotic inducers, including etoposide and staurosporine, and under conditions of serum deprivation. We found that KSHV infection of endothelial cells also increased the ability of these cells to form an in vitro tubular network under conditions of stress and growth factor deprivation. Finally, we show that the nuclear factor-kappaB and PI3K pathways are also required for endothelial tubular network formation. Collectively, these results suggest that KSHV infection of endothelial cells modulates cell signaling pathways and induces cell survival and angiogenesis, thereby contributing to the pathogenesis induced by KSHV.
Figures


Similar articles
-
Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR.Cancer Cell. 2003 Feb;3(2):131-43. doi: 10.1016/s1535-6108(03)00024-2. Cancer Cell. 2003. PMID: 12620408
-
3D culture conditions support Kaposi's sarcoma herpesvirus (KSHV) maintenance and viral spread in endothelial cells.J Mol Med (Berl). 2021 Mar;99(3):425-438. doi: 10.1007/s00109-020-02020-8. Epub 2021 Jan 23. J Mol Med (Berl). 2021. PMID: 33484281 Free PMC article.
-
The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B.Cancer Res. 2001 Mar 15;61(6):2641-8. Cancer Res. 2001. PMID: 11289142
-
Signal Transduction Pathways Associated with KSHV-Related Tumors.Adv Exp Med Biol. 2018;1045:321-355. doi: 10.1007/978-981-10-7230-7_15. Adv Exp Med Biol. 2018. PMID: 29896674 Review.
-
Molecular biology of Kaposi's sarcoma-associated herpesvirus and related oncogenesis.Adv Virus Res. 2010;78:87-142. doi: 10.1016/B978-0-12-385032-4.00003-3. Adv Virus Res. 2010. PMID: 21040832 Free PMC article. Review.
Cited by
-
Cancer angiogenesis induced by Kaposi sarcoma-associated herpesvirus is mediated by EZH2.Cancer Res. 2012 Jul 15;72(14):3582-92. doi: 10.1158/0008-5472.CAN-11-2876. Epub 2012 May 16. Cancer Res. 2012. PMID: 22593192 Free PMC article.
-
KSHV MicroRNAs Repress Tropomyosin 1 and Increase Anchorage-Independent Growth and Endothelial Tube Formation.PLoS One. 2015 Aug 11;10(8):e0135560. doi: 10.1371/journal.pone.0135560. eCollection 2015. PLoS One. 2015. PMID: 26263384 Free PMC article.
-
Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line.J Exp Clin Cancer Res. 2013 Oct 23;32(1):79. doi: 10.1186/1756-9966-32-79. J Exp Clin Cancer Res. 2013. PMID: 24422998 Free PMC article.
-
Kaposi sarcoma-associated herpesvirus g protein-coupled receptor enhances endothelial cell survival in part by upregulation of bcl-2.Ochsner J. 2013 Spring;13(1):66-75. Ochsner J. 2013. PMID: 23532945 Free PMC article.
-
KSHV Induction of Angiogenic and Lymphangiogenic Phenotypes.Front Microbiol. 2012 Mar 30;3:102. doi: 10.3389/fmicb.2012.00102. eCollection 2012. Front Microbiol. 2012. PMID: 22479258 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. - PubMed
-
- Dupin N, Grandadam M, Calvez V, et al. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi’s sarcoma. Lancet. 1995;345:761–2. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS- related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–80. - PubMed
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

