Topotecan: An evolving option in the treatment of relapsed small cell lung cancer
- PMID: 18516270
- PMCID: PMC2387299
Topotecan: An evolving option in the treatment of relapsed small cell lung cancer
Abstract
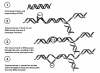
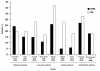
The topoisomerase I inhibitor topotecan is the only single-agent therapy approved for the treatment of recurrent small cell lung cancer (SCLC). Many patients with recurrent SCLC may be predisposed to treatment-related adverse events because of the presence of comorbidities such as advanced age, renal impairment, or extensive prior therapy. In this setting, disease stabilization is considered a treatment benefit, and quality-of-life effects and toxicity profiles of treatments should be considered. The approved regimen of topotecan 1.5 mg/m² on days 1 to 5 of a 21-day cycle is active and has generally mild nonhematologic toxicity and a well-established hematologic toxicity profile characterized by reversible, manageable, and noncumulative neutropenia. Alternative dosing and treatment schedules may lower the incidence of hematologic toxicities while maintaining antitumor efficacy in this patient population. Therefore, topotecan may provide physicians with an effective and versatile therapeutic option for the treatment of patients with relapsed SCLC.
Keywords: relapsed cancer; review; small cell lung cancer; topotecan.
Figures

Similar articles
-
Safety of topotecan in the treatment of recurrent small-cell lung cancer and ovarian cancer.Expert Opin Drug Saf. 2007 Jan;6(1):53-62. doi: 10.1517/14740338.6.1.53. Expert Opin Drug Saf. 2007. PMID: 17181452 Review.
-
Update on the role of topotecan in the treatment of non-small cell lung cancer.Oncologist. 2004;9 Suppl 6:43-52. doi: 10.1634/theoncologist.9-90006-43. Oncologist. 2004. PMID: 15616149 Review.
-
Topotecan in the treatment of relapsed small cell lung cancer patients with poor performance status.Oncologist. 2004;9(2):173-81. doi: 10.1634/theoncologist.9-2-173. Oncologist. 2004. PMID: 15047921 Review.
-
Hematologic safety and tolerability of topotecan in recurrent ovarian cancer and small cell lung cancer: an integrated analysis.Oncologist. 2005 Oct;10(9):686-94. doi: 10.1634/theoncologist.10-9-686. Oncologist. 2005. PMID: 16249347 Review.
-
Emerging role of weekly topotecan in recurrent small cell lung cancer.Oncologist. 2004;9 Suppl 6:25-32. doi: 10.1634/theoncologist.9-90006-25. Oncologist. 2004. PMID: 15616147 Review.
Cited by
-
Phytotherapeutics in Cancer: From Potential Drug Candidates to Clinical Translation.Curr Top Med Chem. 2024;24(12):1050-1074. doi: 10.2174/0115680266282518231231075311. Curr Top Med Chem. 2024. PMID: 38279745 Review.
-
The Efficacy and Safety of Anlotinib in Extensive-Stage Small Cell Lung Cancer: A Multicenter Real-World Study.Cancer Manag Res. 2022 Aug 2;14:2273-2287. doi: 10.2147/CMAR.S364125. eCollection 2022. Cancer Manag Res. 2022. PMID: 35942069 Free PMC article.
-
Function of AP2/ERF Transcription Factors Involved in the Regulation of Specialized Metabolism in Ophiorrhiza pumila Revealed by Transcriptomics and Metabolomics.Front Plant Sci. 2016 Dec 9;7:1861. doi: 10.3389/fpls.2016.01861. eCollection 2016. Front Plant Sci. 2016. PMID: 28018397 Free PMC article.
-
Integrated pipeline for ultrasensitive protein detection in cancer nanomedicine.RSC Adv. 2023 May 15;13(21):14685-14697. doi: 10.1039/d3ra02092d. eCollection 2023 May 9. RSC Adv. 2023. PMID: 37197682 Free PMC article.
-
Current and emerging pharmacotherapies for the treatment of relapsed small cell lung cancer.Clin Med Insights Oncol. 2011;5:223-34. doi: 10.4137/CMO.S5964. Epub 2011 Jul 25. Clin Med Insights Oncol. 2011. PMID: 21836818 Free PMC article.
References
-
- Ardizzoni A. Topotecan in the treatment of recurrent small cell lung cancer: an update. Oncologist. 2004;9(Suppl 6):4–13. - PubMed
-
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol. 1997;15:2090–6. - PubMed
-
- Ardizzoni A, Manegold C, Debruyne C, et al. European organization for research and treatment of cancer (EORTC) 08957 phase II study of topotecan in combination with cisplatin as second-line treatment of refractory and sensitive small cell lung cancer. Clin Cancer Res. 2003;9:143–50. - PubMed
-
- Armstrong D, O’Reilly S. Clinical Guidelines for Managing Topotecan-Related Hematologic Toxicity. Oncologist. 1998;3:4–10. - PubMed
-
- Armstrong DK, Spriggs D, Levin J, et al. Hematologic safety and tolerability of topotecan in recurrent ovarian cancer and small cell lung cancer: an integrated analysis. Oncologist. 2005;10:686–94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

