RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice
- PMID: 18464930
- PMCID: PMC2373419
- DOI: 10.1172/JCI33392
RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice
Abstract
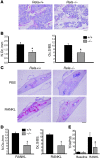
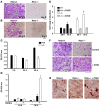
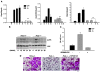
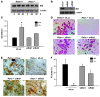
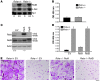
Osteoclasts (OCs) function to reabsorb bone and are responsible for the bone loss associated with inflammatory arthritis and osteoporosis. OC numbers are elevated in most disorders of accelerated bone destruction, reflecting altered rates of precursor differentiation and apoptosis. Both of these processes are regulated by the JNK family of MAP kinases. In this study, we have demonstrated that the NF-kappaB subunit RelA/p65 inhibits JNK-mediated apoptosis during a critical period of commitment to the OC phenotype in response to the cytokine RANKL. This RelA/p65-mediated arrest of cell death led to enhanced OC differentiation. Hence, Rela-/- OC precursors displayed prolonged JNK activation in response to RANKL, and this was accompanied by an increase in cell death that prevented efficient differentiation. Although complete blockade of JNK activity inhibits osteoclastogenesis, both short-term blockade in RelA-deficient cultures and suppression of the downstream mediator, Bid rescued apoptosis and differentiation. These antiapoptotic effects were RelA specific, as overexpression of RelA, but not RelB, blocked apoptosis and rescued differentiation in Rela-/- precursors. Thus, RelA blocks a RANKL-induced, apoptotic JNK-Bid pathway, thereby promoting OC differentiation. Consistent with this, mice lacking RelA/p65 in the hematopoietic compartment were shown to have a deficient osteoclastogenic response to RANKL and were protected from arthritis-induced osteolysis.
Figures
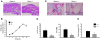
Similar articles
-
Alternative NF-κB Regulates RANKL-Induced Osteoclast Differentiation and Mitochondrial Biogenesis via Independent Mechanisms.J Bone Miner Res. 2015 Dec;30(12):2287-99. doi: 10.1002/jbmr.2584. Epub 2015 Aug 6. J Bone Miner Res. 2015. PMID: 26094846 Free PMC article.
-
Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation.J Biol Chem. 2008 Sep 5;283(36):24546-53. doi: 10.1074/jbc.M800434200. Epub 2008 Jun 19. J Biol Chem. 2008. PMID: 18567579 Free PMC article.
-
JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts.J Bone Miner Res. 2008 Jun;23(6):907-14. doi: 10.1359/jbmr.080211. J Bone Miner Res. 2008. PMID: 18251700
-
12-O-tetradecanoylphorbol-13-acetate (TPA) inhibits osteoclastogenesis by suppressing RANKL-induced NF-kappaB activation.J Bone Miner Res. 2003 Dec;18(12):2159-68. doi: 10.1359/jbmr.2003.18.12.2159. J Bone Miner Res. 2003. PMID: 14672351
-
Epidermal growth factor receptor regulates osteoclast differentiation and survival through cross-talking with RANK signaling.J Cell Physiol. 2008 Nov;217(2):409-22. doi: 10.1002/jcp.21511. J Cell Physiol. 2008. PMID: 18543257
Cited by
-
NF-κB-Mediated Regulation of Osteoclastogenesis.Endocrinol Metab (Seoul). 2015 Mar 27;30(1):35-44. doi: 10.3803/EnM.2015.30.1.35. Endocrinol Metab (Seoul). 2015. PMID: 25827455 Free PMC article. Review.
-
Genetic Polymorphisms of Nuclear Factor-κB Family Affect the Bone Mineral Density Response to Zoledronic Acid Therapy in Postmenopausal Chinese Women.Genes (Basel). 2022 Jul 27;13(8):1343. doi: 10.3390/genes13081343. Genes (Basel). 2022. PMID: 36011257 Free PMC article.
-
Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis.Endocrinol Metab (Seoul). 2023 Oct;38(5):504-521. doi: 10.3803/EnM.2023.501. Epub 2023 Sep 26. Endocrinol Metab (Seoul). 2023. PMID: 37749800 Free PMC article.
-
Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss.J Adv Res. 2023 Nov;53:235-248. doi: 10.1016/j.jare.2023.01.003. Epub 2023 Jan 16. J Adv Res. 2023. PMID: 36657717 Free PMC article.
-
Molecular signaling in bone cells: Regulation of cell differentiation and survival.Adv Protein Chem Struct Biol. 2019;116:237-281. doi: 10.1016/bs.apcsb.2019.01.002. Epub 2019 Feb 4. Adv Protein Chem Struct Biol. 2019. PMID: 31036293 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

