Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN
- PMID: 18337571
- PMCID: PMC2346758
- DOI: 10.1128/JVI.01587-07
Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN
Abstract
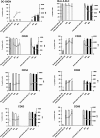
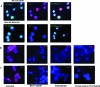
Human herpesvirus 8 (HHV-8) is the etiological agent of Kaposi's sarcoma, primary effusion lymphoma, and some forms of multicentric Castleman's disease. Although latent HHV-8 DNA can be detected in B cells from persons with these cancers, there is little information on the replication of HHV-8 in B cells. Indeed, B cells are relatively resistant to HHV-8 infection in vitro. We have recently shown that DC-SIGN, a C-type lectin first identified on dendritic cells (DC), is an entry receptor for HHV-8 on DC and macrophages. We have also demonstrated previously that B lymphocytes from peripheral blood and tonsils express DC-SIGN and that this expression increases after B-cell activation. Here we show that activated blood and tonsillar B cells can be productively infected with HHV-8, as measured by an increase in viral DNA, the expression of viral lytic and latency proteins, and the production of infectious virus. The infection of B cells with HHV-8 was blocked by the pretreatment of the cells with antibody specific for DC-SIGN or with mannan but not antibody specific for xCT, a cystine/glutamate exchange transporter that has been implicated in HHV-8 fusion to cells. The infection of B cells with HHV-8 resulted in increased expression of DC-SIGN and a decrease in the expression of CD20 and major histocompatibility complex class I. HHV-8 could also infect and replicate in B-cell lines transduced to express full-length DC-SIGN but not in B-cell lines transduced to express DC-SIGN lacking the transmembrane domain, demonstrating that the entry of HHV-8 into B cells is related to DC-SIGN-mediated endocytosis. The role of endocytosis in viral entry into activated B cells was confirmed by blocking HHV-8 infection with endocytic pathway inhibitors. Thus, the expression of DC-SIGN is essential for productive HHV-8 infection of and replication in B cells.
Figures



Similar articles
-
Human Herpesvirus 8 Infects and Replicates in Langerhans Cells and Interstitial Dermal Dendritic Cells and Impairs Their Function.J Virol. 2017 Sep 27;91(20):e00909-17. doi: 10.1128/JVI.00909-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768873 Free PMC article.
-
DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages.J Immunol. 2006 Feb 1;176(3):1741-9. doi: 10.4049/jimmunol.176.3.1741. J Immunol. 2006. PMID: 16424204
-
Analysis of the interaction of Ebola virus glycoprotein with DC-SIGN (dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin) and its homologue DC-SIGNR.J Infect Dis. 2007 Nov 15;196 Suppl 2(Suppl 2):S237-46. doi: 10.1086/520607. J Infect Dis. 2007. PMID: 17940955 Free PMC article.
-
Dendritic cells: key players in human herpesvirus 8 infection and pathogenesis.Front Microbiol. 2014 Aug 28;5:452. doi: 10.3389/fmicb.2014.00452. eCollection 2014. Front Microbiol. 2014. PMID: 25221546 Free PMC article. Review.
-
DC-SIGN: binding receptors for hepatitis C virus.Chin Med J (Engl). 2004 Sep;117(9):1395-400. Chin Med J (Engl). 2004. PMID: 15377434 Review.
Cited by
-
Interaction of human tumor viruses with host cell surface receptors and cell entry.Viruses. 2015 May 22;7(5):2592-617. doi: 10.3390/v7052592. Viruses. 2015. PMID: 26008702 Free PMC article. Review.
-
Kaposi's Sarcoma Associated Herpesvirus Entry into Target Cells.Front Microbiol. 2012 Jan 20;3:6. doi: 10.3389/fmicb.2012.00006. eCollection 2012. Front Microbiol. 2012. PMID: 22319516 Free PMC article.
-
Ephrin Receptor A4 is a New Kaposi's Sarcoma-Associated Herpesvirus Virus Entry Receptor.mBio. 2019 Feb 19;10(1):e02892-18. doi: 10.1128/mBio.02892-18. mBio. 2019. PMID: 30782663 Free PMC article.
-
Infection of primary human tonsillar lymphoid cells by KSHV reveals frequent but abortive infection of T cells.Virology. 2011 Apr 25;413(1):1-11. doi: 10.1016/j.virol.2010.12.036. Epub 2011 Feb 25. Virology. 2011. PMID: 21353276 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin.J Virol. 2008 Dec;82(23):11902-12. doi: 10.1128/JVI.01042-08. Epub 2008 Sep 24. J Virol. 2008. PMID: 18815301 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. - PubMed
-
- Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. - PubMed
-
- Ambroziak, J. A., D. J. Blackbourn, B. G. Herndier, R. G. Glogau, J. H. Gullett, A. R. McDonald, E. T. Lennette, and J. A. Levy. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268582-583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

