Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20
- PMID: 18268035
- PMCID: PMC2271029
- DOI: 10.1084/jem.20071108
Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20
Abstract
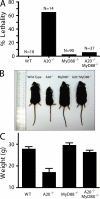
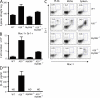
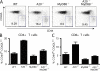
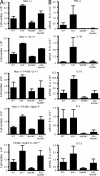
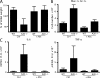
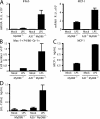
Toll-like receptors (TLRs) on host cells are chronically engaged by microbial ligands during homeostatic conditions. These signals do not cause inflammatory immune responses in unperturbed mice, even though they drive innate and adaptive immune responses when combating microbial infections. A20 is a ubiquitin-modifying enzyme that restricts exogenous TLR-induced signals. We show that MyD88-dependent TLR signals drive the spontaneous T cell and myeloid cell activation, cachexia, and premature lethality seen in A20-deficient mice. We have used broad spectrum antibiotics to demonstrate that these constitutive TLR signals are driven by commensal intestinal flora. A20 restricts TLR signals by restricting ubiquitylation of the E3 ligase tumor necrosis factor receptor-associated factor 6. These results reveal both the severe proinflammatory pathophysiology that can arise from homeostatic TLR signals as well as the critical role of A20 in restricting these signals in vivo. In addition, A20 restricts MyD88-independent TLR signals by inhibiting Toll/interleukin 1 receptor domain-containing adaptor inducing interferon (IFN) beta-dependent nuclear factor kappaB signals but not IFN response factor 3 signaling. These findings provide novel insights into how physiological TLR signals are regulated.
Figures

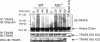
Similar articles
-
Mycobacterium fortuitum induces A20 expression that impairs macrophage inflammatory responses.Pathog Dis. 2016 Apr;74(3):ftw015. doi: 10.1093/femspd/ftw015. Epub 2016 Mar 2. Pathog Dis. 2016. PMID: 26940588
-
B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice.Blood. 2011 Feb 17;117(7):2227-36. doi: 10.1182/blood-2010-09-306019. Epub 2010 Nov 18. Blood. 2011. PMID: 21088135
-
The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals.Immunity. 2008 Mar;28(3):381-90. doi: 10.1016/j.immuni.2008.02.002. Immunity. 2008. PMID: 18342009 Free PMC article.
-
Toll-like receptor signaling in hematopoietic homeostasis and the pathogenesis of hematologic diseases.Front Med. 2015 Sep;9(3):288-303. doi: 10.1007/s11684-015-0412-0. Epub 2015 Aug 22. Front Med. 2015. PMID: 26297301 Review.
-
TLRs control hematopoiesis during infection.Eur J Immunol. 2013 Oct;43(10):2526-33. doi: 10.1002/eji.201343833. Eur J Immunol. 2013. PMID: 24122753 Free PMC article. Review.
Cited by
-
The ubiquitylation of IL-1β limits its cleavage by caspase-1 and targets it for proteasomal degradation.Nat Commun. 2021 May 11;12(1):2713. doi: 10.1038/s41467-021-22979-3. Nat Commun. 2021. PMID: 33976225 Free PMC article.
-
Pathogenetic importance and therapeutic implications of NF-κB in lymphoid malignancies.Immunol Rev. 2012 Mar;246(1):359-78. doi: 10.1111/j.1600-065X.2012.01105.x. Immunol Rev. 2012. PMID: 22435566 Free PMC article. Review.
-
The microbiome in infectious disease and inflammation.Annu Rev Immunol. 2012;30:759-95. doi: 10.1146/annurev-immunol-020711-074937. Epub 2012 Jan 6. Annu Rev Immunol. 2012. PMID: 22224764 Free PMC article. Review.
-
Effects of gut microbiota and time of treatment on tissue levels of green tea polyphenols in mice.Biofactors. 2018 May 8:10.1002/biof.1430. doi: 10.1002/biof.1430. Online ahead of print. Biofactors. 2018. PMID: 29740891 Free PMC article.
-
A20 is critical for the induction of Pam3CSK4-tolerance in monocytic THP-1 cells.PLoS One. 2014 Jan 28;9(1):e87528. doi: 10.1371/journal.pone.0087528. eCollection 2014. PLoS One. 2014. PMID: 24489933 Free PMC article.
References
-
- Hooper, L.V., and J.I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science. 292:1115–1118. - PubMed
-
- Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. - PubMed
-
- Rakoff-Nahoum, S., L. Hao, and R. Medzhitov. 2006. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 25:319–329. - PubMed
-
- Ting, J.P., D.L. Kastner, and H.M. Hoffman. 2006. CATERPILLERs, pyrin and hereditary immunological disorders. Nat. Rev. Immunol. 6:183–195. - PubMed
-
- Takeda, K., and S. Akira. 2004. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34:73–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

