BRCA1 regulates human mammary stem/progenitor cell fate
- PMID: 18230721
- PMCID: PMC2234204
- DOI: 10.1073/pnas.0711613105
BRCA1 regulates human mammary stem/progenitor cell fate
Abstract
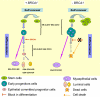
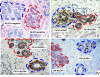
Although it is well established that women with germ-line mutations in the BRCA1 gene have a greatly increased lifetime incidence of breast and ovarian cancer, the molecular mechanisms responsible for this tissue-specific carcinogenesis remain undefined. The majority of these breast cancers are of the basal-like phenotype characterized by lack of expression of ER, PR, and ERBB2. Because this phenotype has been proposed to resemble that of normal breast stem cells, we examined the role of BRCA1 in human mammary stem cell fate. Using both in vitro systems and a humanized NOD/SCID mouse model, we demonstrate that BRCA1 expression is required for the differentiation of ER-negative stem/progenitor cells to ER-positive luminal cells. Knockdown of BRCA1 in primary breast epithelial cells leads to an increase in cells displaying the stem/progenitor cell marker ALDH1 and a decrease in cells expressing luminal epithelial markers and estrogen receptor. In breast tissues from women with germ-line BRCA1 mutations, but not normal controls, we detect entire lobules that, although histologically normal, are positive for ALDH1 expression but are negative for the expression of ER. Loss of heterozygosity for BRCA1 was documented in these ALDH1-positive lobules but not in adjacent ALDH1-negative lobules. Taken together, these studies demonstrate that BRCA1 plays a critical role in the differentiation of ER-negative stem/progenitor cells to ER-positive luminal cells. Because BRCA1 also plays a role in DNA repair, our work suggests that loss of BRCA1 may result in the accumulation of genetically unstable breast stem cells, providing prime targets for further carcinogenic events.
Conflict of interest statement
Conflict of interest statement: M.S.W. has financial holdings in and is a scientific advisor for OncoMed Pharmaceuticals.
Figures




Similar articles
-
Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice.Breast Cancer Res. 2011 Mar 11;13(2):R30. doi: 10.1186/bcr2850. Breast Cancer Res. 2011. PMID: 21396117 Free PMC article.
-
Expression of aldehyde dehydrogenase 1 as a marker of mammary stem cells in benign and malignant breast lesions of Ghanaian women.Cancer. 2013 Feb 1;119(3):488-94. doi: 10.1002/cncr.27737. Epub 2012 Aug 28. Cancer. 2013. PMID: 22930220 Free PMC article.
-
Expression of the stem cell marker ALDH1 in BRCA1 related breast cancer.Cell Oncol (Dordr). 2011 Feb;34(1):3-10. doi: 10.1007/s13402-010-0007-3. Epub 2011 Feb 19. Cell Oncol (Dordr). 2011. PMID: 21336637 Free PMC article.
-
The cell of origin of BRCA1 mutation-associated breast cancer: a cautionary tale of gene expression profiling.J Mammary Gland Biol Neoplasia. 2011 Apr;16(1):51-5. doi: 10.1007/s10911-011-9202-8. Epub 2011 Feb 19. J Mammary Gland Biol Neoplasia. 2011. PMID: 21336547 Review.
-
NF-κB at the Crossroads of Normal Mammary Gland Biology and the Pathogenesis and Prevention of BRCA1-Mutated Breast Cancer.Cancer Prev Res (Phila). 2018 Feb;11(2):69-80. doi: 10.1158/1940-6207.CAPR-17-0225. Epub 2017 Nov 3. Cancer Prev Res (Phila). 2018. PMID: 29101208 Review.
Cited by
-
BRCA1 Protein Expression Level and CD44(+)Phenotype in Breast Cancer Patients.Cell J. 2011 Fall;13(3):155-62. Epub 2011 Sep 23. Cell J. 2011. PMID: 23508738 Free PMC article.
-
Interplay between BRCA1 and RHAMM regulates epithelial apicobasal polarization and may influence risk of breast cancer.PLoS Biol. 2011 Nov;9(11):e1001199. doi: 10.1371/journal.pbio.1001199. Epub 2011 Nov 15. PLoS Biol. 2011. PMID: 22110403 Free PMC article.
-
The Expression and Clinical Outcome of pCHK2-Thr68 and pCDC25C-Ser216 in Breast Cancer.Int J Mol Sci. 2016 Oct 28;17(11):1803. doi: 10.3390/ijms17111803. Int J Mol Sci. 2016. PMID: 27801830 Free PMC article.
-
Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype.Environ Health Perspect. 2009 Dec;117(12):1847-52. doi: 10.1289/ehp.0900999. Epub 2009 Aug 13. Environ Health Perspect. 2009. PMID: 20049202 Free PMC article.
-
Environmental exposures, stem cells, and cancer.Pharmacol Ther. 2019 Dec;204:107398. doi: 10.1016/j.pharmthera.2019.107398. Epub 2019 Jul 31. Pharmacol Ther. 2019. PMID: 31376432 Free PMC article. Review.
References
-
- Wicha MS, Liu S, Dontu G. Cancer Res. 2006;66:1883–1890. - PubMed
-
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Cancer Res. 2005;65:10946–10951. - PubMed
-
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M, et al. Cancer Res. 2005;65:9328–9337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

